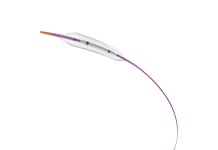
Nitinotes, a medical device company transforming the treatment of obesity, today announced it has received CE Mark approval for the EndoZip System, the first fully automated suturing platform for endoscopic sleeve gastroplasty (ESG). The milestone clearance enables Nitinotes to begin commercialisation across the European Union and other CE Mark-accepting markets.
Obesity is one of the most urgent global health challenges, affecting more than 650 million adults worldwide. Current treatment pathways leave a significant gap between medical management such as GLP-1 therapy and invasive bariatric surgery. ESG is emerging as a minimally invasive alternative, addressing patients with obesity (BMI 30–40 kg/m²) who have not achieved success with conservative measures such as diet, exercise, or medication.
Related: Medical Microinstruments wins FDA IDE for robotic microsurgery for Alzheimer’s
By automating suturing, EndoZip transforms ESG into a scalable, single-physician, fast, outpatient procedure with a short learning curve. Unlike current manual suturing systems that demand two physician operators, longer procedure times, and advanced endoscopic skills, EndoZip delivers unmatched efficiency, reproducibility, and economics – democratizing ESG and expanding access to bariatric surgeons while setting a new standard for care.
“CE Mark approval of EndoZip is a pivotal milestone for Nitinotes,” said Lloyd Diamond, Chief Executive Officer of Nitinotes. “This achievement validates the safety and performance of our technology and paves the way for commercialization in Europe. With EndoZip, we have the potential to dramatically expand patient access to a safe, durable, minimally invasive obesity treatment while creating significant value for providers and healthcare systems.”
Nitinotes will launch EndoZip in select European centers of excellence, building clinical adoption and real-world data to support broader market penetration. In parallel, the company is preparing for its pivotal U.S. IDE trial, which will support FDA submission and entry into the world’s largest obesity treatment market.