Virtuoso Surgical announced it received FDA breakthrough device designation for bladder lesion removal with its surgical robot.
The breakthrough nod covers bladder lesion removal via en bloc excision with the Virtuoso robot. Nashville, Tennessee–based Virtuoso Surgical says it positions the company “at the forefront of early diagnosis and innovative bladder care.”
Related: FDA clears Techsomed BioTraceIO360 for kidney ablation
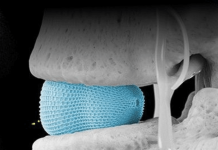
Using a surgeon-controlled system with needle-sized robotic arms, the Virtuoso device can significantly enhance the precision and dexterity of minimally invasive surgery performed via rigid endoscopes. That endoscope comes in at less than half the diameter of a U.S. dime. The scope is smaller than current endoscope hardware, the company says, and the manipulators are 1mm in diameter. It also features a camera, tissue grasper, retractor, tissue snare, laser aiming manipulator and electrosurgical tools.
The company says en bloc resection preserves the integrity of the pathological specimen and increases the accuracy of cancer staging. This results in more definitive diagnosis compared to the standard transurethral resection approach.
CEO Dr. Duke Herrell announced the successful first-in-human clinical cases with the company’s robotic endoscopy system in May. The company now seeks to push for FDA approval and to explore the use of the technology in urology and later in other specialties. Those could include gynecology, pulmonology, otolaryngology and neurosurgery.
“Virtuoso is unlike any surgical robot available today, and our vision is to provide surgeons with unprecedented dexterity and control in rigid endoscopic surgery, which is a distinct set of procedures and approaches that have not yet benefited from robotics,” Herrell said. “Virtuoso is starting with the removal of bladder lesions, the first critical step in diagnosing, staging and determining the management for cancerous lesions.”