Seventh Sense Biosystems, (7SBio), developers of a push-button blood collection device, has received CE mark approval from the European Commission for its TAP II blood collection device. 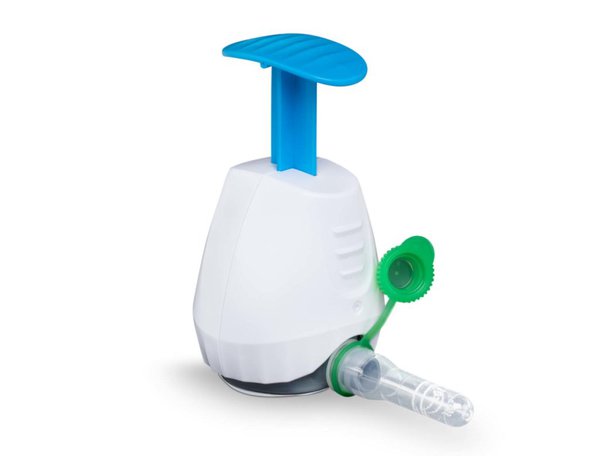
7SBio
Rick Bente, CEO of 7SBio, said: “We’re very excited to bring TAP II into the European marketplace with the CE marking approval. This clearance, and the support from our investors, paves the way for our innovative blood collection technology to enter the homes of our European customers and to provide easier access to healthcare services. Seventh Sense’s vision to revolutionise the way the world draws blood is now ready for the world’s stage, and with the COVID-19 pandemic transforming how healthcare is delivered to consumers, it could not come at a better time.”
Seventh Sense has raised over $80 million to date in pursuit of this mission and continues to advance its proprietary micro-needle technology used in its CE-marked and FDA-approved TAP device. The second-generation TAP II device expands the volume of blood that can be collected while delivering standardised and detachable serum and plasma collection containers that fit seamlessly into laboratory workflows.
The process is simple. A patient places TAP II on their upper arm and presses a button on the device to begin blood collection. The process to collect blood takes only two to three minutes. After collection is complete, the device is sent back to a lab for analysis.

Chris Moriarty, CEO at Affinity Biomarker Labs, said: “We believe that the simple blood collection process enabled by TAP II not only will help shape telehealth in general, but it also helps to keep the public safer during the COVID-19 pandemic. As a lab providing both direct-to-patient testing and lab services for consumer healthcare companies, our patients and customers appreciate the ease and more comfortable experience of using TAP II, and the convenience of safe and effective blood collection without leaving home.”
The current standard of care for blood collection is phlebotomy, which requires medical professionals, large needles, and in-person appointments to collect blood. However, TAP II can be used in place of traditional venipuncture or fingerstick blood collection for many applications in both professional and at-home settings.
With CE mark clearance for TAP II, 7SBio’s mission to make blood collection simple, convenient, and more comfortable has arrived for European customers.




