Funds to Support Completion of Clinical Program Through Commercialization of Novel Device Designed to Prevent Amputations in Patients with Chronic Limb-Threatening Ischemia
LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), today announced it has closed a $40 million (€36 million) oversubscribed Series D financing round. New investors Longitude Capital, Soleus Capital Management and an undisclosed strategic investor joined the round along with current major shareholders of the company: Sofinnova Partners, through its Crossover Strategy fund; Bpifrance, the French sovereign investment bank; and Balestier, a Singaporean family fund.
“We are truly impressed by the clinical results LimFlow has delivered for patients who have exhausted all other treatment options. We are excited about the technology’s potential to transform the way CLTI patients are treated, and in turn, transform the quality of their lives”
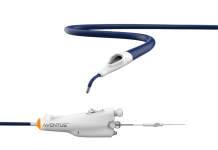
The LimFlow System, designated by the FDA as a Breakthrough Technology, uses a minimally-invasive family of transcatheter products designed to optimize perfusion of the critically ischemic foot, potentially avoiding major amputation, resolving pain and promoting wound healing. Proceeds from the Series D financing will fund follow-up for the PROMISE II U.S. pivotal trial necessary to obtain FDA approval and will support the commercialization of the LimFlow System for deep vein arterialization.
The company recently completed enrollment in PROMISE II, the U.S. pivotal trial of the LimFlow System. It also completed enrollment in CLariTI, a natural history study of high-risk and no-option CLTI patients, intended to shed light on outcomes from patients treated with the current standard of care. Over the last year, the company also integrated the second-generation of its LimFlow System into its clinical program, and reported positive two-year data from its PROMISE I U.S. feasibility study.
“We are thankful for the support of so many industry-leading investors, both new and existing, who recognize the potential of the LimFlow System to address a major clinical need in saving patients from amputation,” said LimFlow CEO Dan Rose. “We look forward to sharing results from our pivotal trial later this year, and to the prospect of being able to make the LimFlow system commercially available as early as next year to the patients in the U.S. and Europe who desperately need it.”
“We are truly impressed by the clinical results LimFlow has delivered for patients who have exhausted all other treatment options. We are excited about the technology’s potential to transform the way CLTI patients are treated, and in turn, transform the quality of their lives,” said Maxwell Bikoff, Principal at Longitude Capital.
“The LimFlow management team has achieved key milestones and financing needed to successfully bring the LimFlow System to market. We are enthusiastic about continuing to work with the company to help make this game-changing technology accessible to patients in the near future,” said Kinam Hong, MD, Partner at Sofinnova Partners.
About Chronic Limb-Threatening Ischemia (CLTI)
CLTI is the most severe form of PAD and often occurs in patients suffering from coronary artery disease, diabetes, obesity, high cholesterol and/or high blood pressure. Patients with CLTI often experience profound, chronic pain and develop festering wounds or infections that lead to major limb amputation, an event closely associated with increased mortality and reduced quality of life. To relieve the symptoms of CLTI, patients today are treated primarily with angioplasty or open bypass surgery. In many late-stage patients, however, neither option is feasible due to extensive disease in the target arteries or other anatomical constraints.
About LimFlow and the LimFlow System
LimFlow is a private, venture-backed medical device company transforming the treatment of chronic limb-threatening ischemia, a growing clinical need in the face of the prevalence of diabetes, heart disease, kidney disease and an aging population.
When all other therapeutic options have been exhausted and a CLTI patient is facing major amputation, the minimally-invasive LimFlow system is designed to bypass blocked arteries in the leg and deliver oxygenated blood back into the foot via the veins. For many patients, restoring perfusion in the lower limbs resolves chronic pain, improves quality of life, enables wound healing, and prevents major amputation.
For more information, visit www.limflow.com.
CAUTION: The LimFlow technology is approved for investigational use only in the United States. The LimFlow System received the CE Mark and is currently available commercially in Europe. The LimFlow System has not been approved for sale in the USA, Canada, or Japan.