Recovering from a stroke is a long, arduous process, requiring months or even years of speech, physical and occupational therapy to regain even a semblance of a patient’s previous abilities. During those sessions, the brain is essentially healing itself, an imperfect process that can result in chronic health conditions for at least half of stroke survivors.
BrainQ, however, is developing a quicker, more effective fix for stroke and other neurological conditions—one that just earned the Israeli startup an outpouring of $40 million in venture funding.
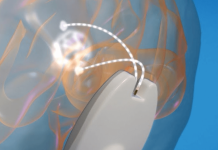
BrainQ’s system relies on electromagnetic field therapy to stimulate the neural networks governing motor function and other neurological activity, delivering low-frequency treatment to repair any damage to those neural networks and resume normal brain function.
The new investment will help cover the costs of a clinical trial that BrainQ is planning to launch in several U.S. hospitals. The study will aim to demonstrate the technology’s ability to lessen the long-term effects of stroke, “significantly increasing the window of opportunity for reducing disability and enhancing recovery potential,” according to Yotam Drechsler, BrainQ’s CEO and co-founder.
The funding round was led by Hanaco Ventures, with additional participation from Dexcel Pharma and Peregrine Ventures.
Along with raking in the $40 million financing—which brings the company’s lifetime funding over the $50 million mark—BrainQ also added to its board of directors. The newest member is Stacey Pugh, who boasts plenty of medtech expertise from both her current role as chief commercial officer of Butterfly Network as well as the six years she spent as a vice president of Medtronic’s neurovascular business.
“While I’ve had the privilege to be closely involved in some of the largest efforts for influencing stroke care, when it comes to long-term recovery there is still much that can be done to restore patients’ health and abilities,” Pugh said. “I’ve been watching BrainQ’s development of their technology and clinical data for a while, and I believe their therapies have the potential to make a real difference for stroke sufferers and their families.”
BrainQ’s plans to begin clinical trials of the technology come not long after it received the FDA’s breakthrough-device designation. Since being granted the classification in February of this year, BrainQ said it has been working closely with the agency to shuttle its technology down an expedited development pathway.
The company’s system uses a noninvasive wearable device to collect brainwave readings. Machine learning algorithms then analyze those readings to map out a low-intensity, low-frequency electromagnetic field that mimics the normal activity of the brain’s neural networks to repair any broken or blocked pathways within those networks.
The entire system is connected to the cloud, allowing patients to administer their own neuromodulation therapy at home while their care teams remotely monitor the sessions via mobile app.
Are you hiring?
The FDA offered up its breakthrough designation based on early results of a small study of the device, which found that after eight weeks of treatment with the technology, nearly 80% of the 25 participants had a score of 1 or 0 on the modified Rankin Scale for post-stroke disability, indicating either minor symptoms or none at all.
In all, more than 90% of the patients in the treatment group saw their modified Rankin Scale scores improve by two or more points after the treatment period, for an average improvement of two and a half points, compared to a drop of just over one point in the control group.