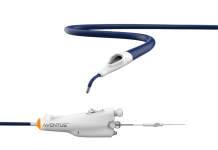
Danish medtech company Neurescue has gained a European CE mark for its catheter to address non-shockable cardiac arrest.
The company’s catheter is used in conjunction with a handheld control unit to temporarily inflate a soft balloon via the femoral artery into the descending aorta to promote the redirection of blood flow towards the heart and brain during cardiopulmonary resuscitation (CPR).
Related: Lifeward wins CE mark for ReWalk 7 personal endoskeleton
According to Neurescue, the technique, known as aortic balloon occlusion (ABO), serves to boost central blood flow within one minute of deployment to restore a heartbeat in cardiac arrest patients, thereby carving out ‘bridging’ time to get patients access to ventricular assist or coronary interventional therapies.
Neurescue’s CEO and founder Habib Frost commented: “This is a breakthrough approval for millions of cardiac arrest patients annually.
“The CE mark approval is more than a regulatory milestone. It bridges a gap for millions of patients facing the most critical life-threatening condition.”
Research by the US Centers for Disease Control and Prevention (CDC) indicates that cardiac arrest results in around 436,000 deaths in the US annually, with 90% of out-of-hospital events proving fatal.
Non-shockable cardiac arrest, in which patients have no electrical activity or pulseless electrical activity (PEA), cannot be treated with defibrillators and is associated with higher mortality rates compared to cardiac arrest cases wherein shockable rhythms like ventricular fibrillation or pulseless ventricular tachycardia are present.
Up to 81% of in-hospital cardiac arrests in the US present with a non-shockable rhythm, such as pulseless electrical activity (PEA), as per the American Heart Association’s (AHA) Get With The Guidelines – Resuscitation (GWTG-R) programme, a registry that aims to improve patient outcomes following in-hospital cardiac arrest (IHCA) through evidence-based guidelines.
In 2021, Neurescue’s catheter received 510(k) clearance from the US Food and Drug Administration (FDA) for emergency control of haemorrhage and an investigational device exemption (IDE) for use in cardiac arrest.
Neurescue is poised to initiate a study [NCT06793033], which will compare the clinical safety and performance of Advanced Cardiovascular Life Support (ACLS) versus ACLS in combination with its ABO catheter in subjects with cardiac arrest, as per a listing on Clinicaltrials.gov.