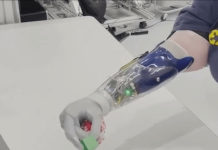
The system provides visual feedback regarding the location of the probe and reduces radiation exposure during surgery.

Spinal instrumentation firm Waypoint Orthopedics has submitted an application seeking 510(k) clearance from the US Food and Drug Administration (FDA) for its smart bone awl, known as Waypoint GPS.
A fully disposable, single-use pedicle probe, Waypoint GPS has been designed for use during pedicle screw pilot hole drilling.
It provides real-time visual feedback to physicians regarding the cutting-edge location of the probe.
This helps physicians identify the probe’s location in the pedicle and reduces the use of radiation to avoid cortical breaches during traditional surgery.
Using proprietary colour sensing technology, the system provides information that is required for determining the difference between cancellous and cortical bone, helping to prevent cortical breaches.
The technology wirelessly displays real-time visual feedback on an app-based operating system.
The feedback indicates a colour change at the tip of the probe, which can be due to contact of the tip with soft tissues and possible vertebral cortex perforation.
RELATED: DyAnsys receives US FDA approval for First Relief neurostimulation device
The company stated that the Waypoint GPS looks, feels and acts like a standard bone awl and makes vertebral fixation potentially safer and more efficient for physicians in the operating room.
Waypoint Orthopedics president and CEO Jeffrey O’Donnell said: “The Waypoint Orthopedics team has worked tirelessly over the last 18 months to develop and test the Waypoint GPS, in anticipation of this submission. I am incredibly proud of all the hard work.
“We are excited to work with the FDA in bringing this much-needed tool to the operating room, enhancing the surgeon’s confidence during the cannulation of each pedicle, while potentially reducing the amount of ionising radiation during a case.
“We believe in surgeon-designed products and have had tremendous input and guidance from our world-class Surgeon Advisory Board throughout this process.”
The company noted that the new system is indicated for use in open and percutaneous (MIS) surgical approaches to the spine.
Upon receipt of the FDA approval, which is expected later in the year, Waypoint Orthopedics plans to transfer its technology to a commercial entity.