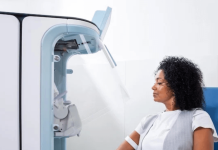
THERACLION has announced the results of the first SONOVEIN® trial in the United States, a major milestone towards receiving FDA approval and accessing the US varicose veins market.
100% success rate in the primary end point and 95% abolition of venous reflux
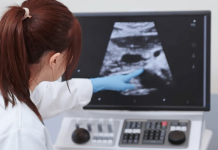
The definitive results show a 100% feasibility result, the primary end point of the trial. Patients did not experience any serious adverse event. Abolition of venous reflux, as measured by duplex ultrasound, was achieved in 95% of cases.
The study compliance was 100%, meaning all 80 visits were completed and timely, none lost to follow-up or missed visits.
RELATED: Theraclion Announces First Patients Treated With SONOVEIN® in the US
A total of 20 patients were enrolled in this clinical trial approved by the FDA (Food & Drug Administration) in early 2022. Internationally recognized KOLs Dr Steve Elias, Dr Antonios Gasparis and Dr Nicos Labropoulos conducted this first trial in New Jersey, United States.
Dr Steve Elias declared: “I am happy to have finished the first FDA-approved trial with SONOVEIN in the United States. More importantly, the results are as good – if not a little bit better – than some of the results with the more traditional technologies. 95% of patients had a successful healing of the abnormal vein. We treat people who have abnormal veins, and not just the veins. Most important, all patients felt better. SONOVEIN really is the next evolution of a completely non-invasive, transcutaneous way of treating vein diseases. We look forward to being able to offer this to our patients.”
Theraclion’s Chief Medical Officer and Veins Vice President Dr Michel Nuta said: “These very robust results generated by first class KOLs are the launchpad for our pivot multi-center FDA study, which will start shortly. The USA patients are needing the access to an extra corporeal incision-free varicose veins treatment, without catheters or foreign chemical products insertion.”