Spectrum Solutions has received CE Mark approval to sell and distribute its SDNA saliva collection devices to conduct IVD molecular diagnostic testing in Europe.
Spectrum Solutions has received CE Mark approval to sell and distribute its SDNA saliva collection devices to conduct IVD molecular diagnostic testing in Europe.
The Spectrum SDNA-2000 model can be used with over the counter at-home testing kits, while SDNA-3000 model can be used for prescription-only molecular IVD diagnostic testing.
Spectrum Solutions CEO Stephen Fanning said: “Receiving CE Mark approval for the SDNA-2000 and SDNA-3000 is a major milestone for our company and this offers physicians, healthcare organisations, governments, and individuals, needing repeat IVD diagnostic testing, an easy and pain-free option.
“At-home bio-sample self-collection means staying home when you don’t feel good and limiting exposure to other harmful illnesses for those most at risk.”
In October last year, the US Food and Drug Administration (FDA) granted emergency use authorisation (EUA) to Spectrum Solutions’ SDNA-1000 saliva collection system for saliva-based Covid-19 testing.
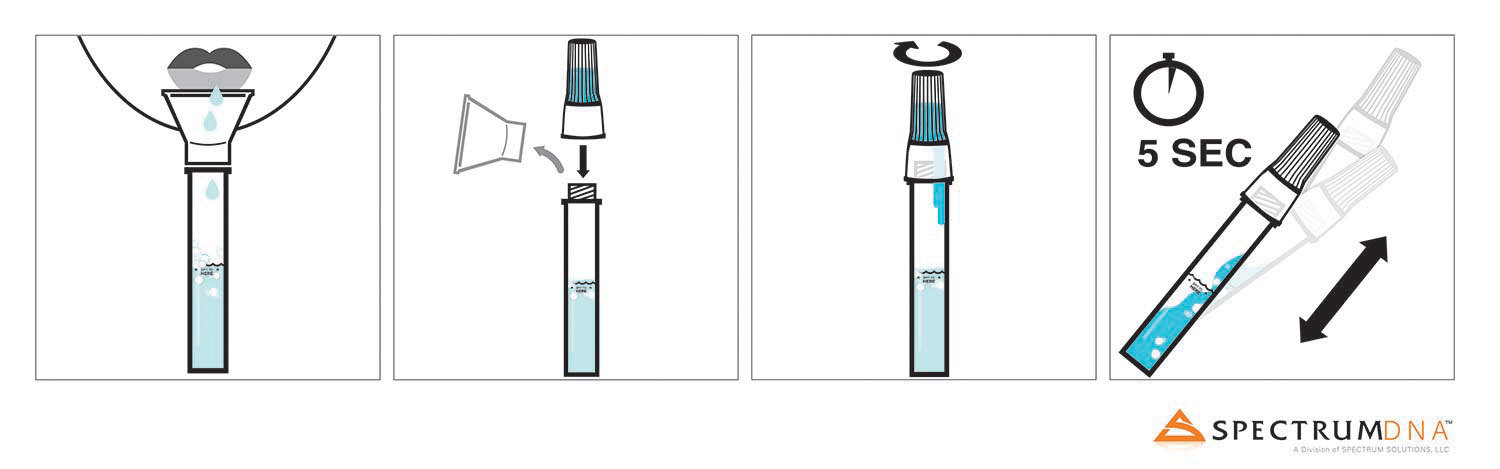
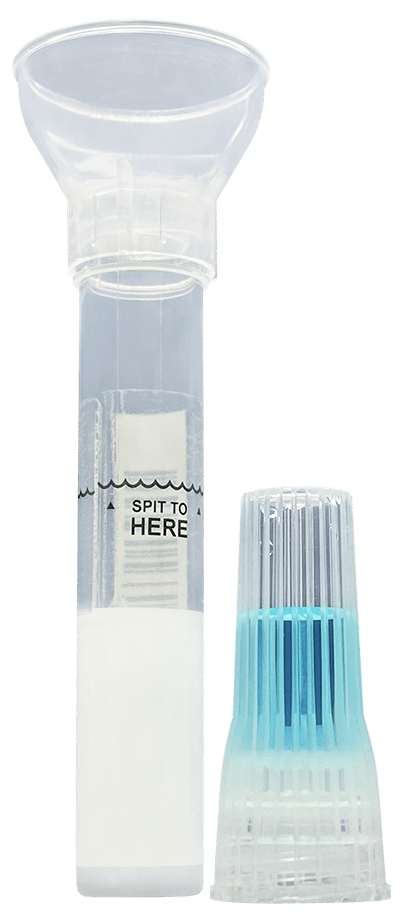
The self-contained saliva collection kit offers critical sample consistency, as well as suspends and neutralises viral RNA transcripts, inactivating the live Sars-CoV-2 virus.
Spectrum Solutions noted that the bio-samples obtained using the SDNA saliva collection system have consistently shown increased levels of testing accuracy and deliver the testing biomaterial, which is safe.
In addition, the device can identify the life cycle stage of infection and offers more than ten days of post-collection stability without affecting the efficacy of the sample.
With its easily usable design, the device was engineered to help in unsupervised self-collection of the testing sample to eradicate user collection errors.
In August last year, US-based Accumen has announced a partnership agreement with Spectrum Solutions to offer Covid-19 saliva testing kits in the US.


