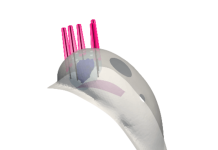
Liquet Medical Inc., a pioneering medical device company committed to advancing patient care through innovative technologies, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Versus™ Catheter.
This innovative medical device is set to enhance the treatment of pulmonary artery blood clots by offering real-time pulmonary artery pressure measurements, empowering clinicians to optimize treatment based on individual patient responses. This novel technology enables a new treatment category called “Hemodynamics-Led Thrombolysis (HLT).”
Related: FemPulse wins FDA IDE for for overactive bladder neuromod
“The Versus Catheter will allow for a more data-centric approach to each individual patient suffering from blood clots in their lungs,” said Luke Wilkins, MD, Interventional Radiologist at UVA Health. “Hemodynamic-led thrombolytic therapy is an opportunity to potentially improve patient outcomes, and we are looking forward to studying this device.”
Key Features and Benefits of the Versus™ Catheter:
– Real-Time Pulmonary Artery Pressure Monitoring: Provides physicians live data to assess patient hemodynamics before and during therapy to optimize treatment decisions.
– Dual-Tip Telescoping Catheter: Enables simultaneous treatment of both lungs with a single device, offering access site alternatives to the physician, and improving healthcare efficiency.
– Flexible Placement: Standard guidewire technique or flow-directed balloon placement.
“FDA 510(k) clearance is a significant milestone for Liquet Medical and enables the commercial release of the Versus Catheter to hospitals and clinics across the U.S. We are thrilled to announce FDA clearance for the Versus™ Catheter, a groundbreaking advancement in the treatment of pulmonary artery blood clots,” said John Schindler, CEO of Liquet Medical. “We believe this innovation has the potential to significantly improve patient outcomes, minimize risks, and reduce healthcare costs.