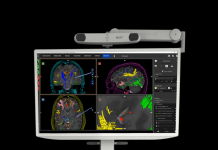
Biotronik won FDA approval for its Renamic Neo programmer for implanted cardiac rhythm management devices this year, thanks to some help from an electronic components supplier.
The Lake Oswego, Oregon-based device developer designed the Renamic Neo for implantable cardioverter defibrillators, pacemakers and implantable cardiac monitors. The control unit wirelessly communicates with the implanted device and allows the physician or patient to read out information such as battery status, heart rate and other important parameters. The control unit can also wirelessly program the implant.
The Renamic Neo’s wireless communication relies on a low-power radio signal, so there can be no interference in the signal’s frequency range. Electromagnetic compatibility (EMC) was an important part of the development of the device, which uses components with low electromagnetic radiation made by Schurter.
Schurter said the device is the world’s first for reading and programming these implants completely wirelessly. The electronics supplier offered its perspective on the Renamic Neo’s development and design.
“Thanks to the knowledge we have of EMC, combined with our expertise in the field of control panels, we were able to make a significant contribution in this project,” Schurter Account Manager Willem Zoeten said in comments shared with Medical Design & Outsourcing. “Biotronik has a lot of knowledge about EMC. We brought in a lot of expertise to come up with a producible product. Based on the requirements set by Biotronik, we redesigned the entire PCB … to ensure that you are exposed to as little emission as possible.”
Schurter and Biotronik tested controller types in an emission-free EMC chamber at Biotronik, Schurter Product Development Engineer Paul Berning said.
“We also tested and optimized the other components, such as the LCD display, in this way,” he said. “Finally, we developed prototypes with the best-performing components, which were also tested at Biotronik. Particularly good was the verdict.”
Biotronik asked Schurter to include an EMC test in the standard production process for the Renamic Neo.
“Biotronik wanted to officially measure that their products met the medical EMC standard EN 60601-1-2 and supplied the equipment to do so,” he said. “There is now a shielded cabinet here, where no radiation can penetrate from the outside. The product is placed in the cabinet, connected with cabling and switched on. An antenna in the cabinet captures all signals around the frequency in question, and these go through a coax cable to a spectrum analyzer outside the cabinet, which measures the signals. An algorithm determines an average value for these signals, which must lie between 3 and 20 dB.”
The collaboration has also reduced production costs by more than 20% per unit in the first two years, Zoeten said.
“Now it is a question of fine-tuning the production process, where we look in particular at the cost structure,” he said. “Is there anything else we can improve in terms of production technology? Can we use other materials or production methods to reduce costs?”
Production is currently at pre-series volume with an eventual target of a few thousand per year.
Are you Hiring?