The eXciteOSA strengthens tongue muscles through electrical stimulation and comes with a companion app for tracking and sharing progress.

The FDA has granted De Novo authorization to what will be the first device treatment for obstructive sleep apnea (OSA) that is used while the patient is awake.
Called eXciteOSA, the prescription tongue muscle stimulation device is intended for those aged 18 years or older to reduce mild sleep apnea and snoring. Patients use the device for 20 minutes once per day over a six-week period, and afterward once weekly.
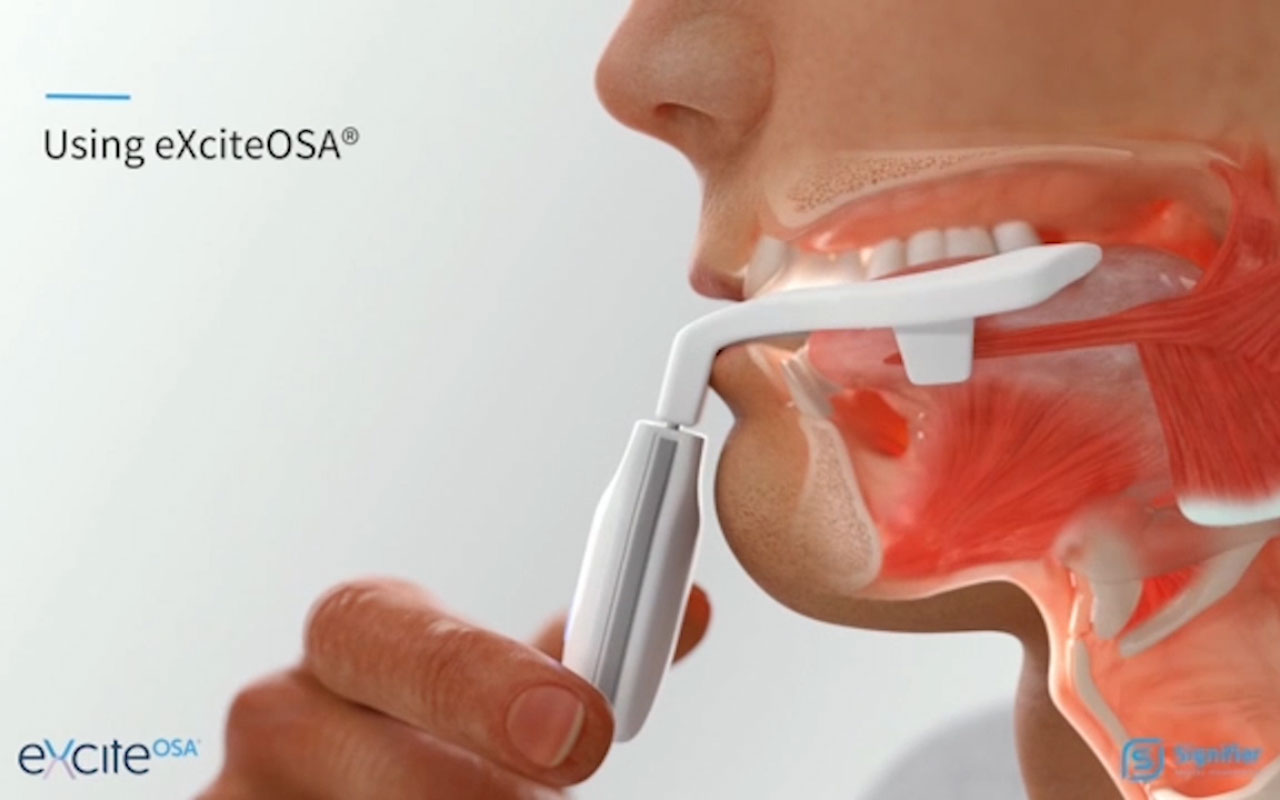
To use it, patients place a silicone mouthpiece with four electrodes around their tongue, manufacturer Signifier Medical Technologies describes on the product’s website. The device’s stimulations strengthen the tongue’s muscles to help it prevent obstructions during the night.
Supporting the mouthpiece is the eXciteOSA’s rechargeable control unit, which powers the mouthpiece, and a corresponding smartphone app that patients can use to monitor the progress of their therapy, track changes in their snoring over time, set treatment reminders and share data with their doctor.eXciteOSA is not intended for those who have or are suspected of having higher severity OSA, according to the FDA’s announcement, and patients interested in the device should first receive a comprehensive dental examination. It’s contraindicated for patients with pacemakers or implanted pacing leads, patients who are pregnant, patients with ulcerations in or around the mouth and patients with various dental and orthodontic hardware.
OSA can lead to a number of long-term health impacts, including heart attacks, glaucoma, diabetes, cancer, and cognitive and behavioral health disorders, according to the FDA’s announcement. A 2013 American Journal of Epidemiology paper estimated that 26% of U.S. adults aged 30 to 70 years have some degree of sleep apnea, while a more recent Lancet Respiratory Medicine study estimated the worldwide burden of mild to severe OSA among adults ages 30 to 69 to be 936 million.
The agency said that its De Novo decision was based on the benefits of the treatment among a body of 115 patients with snoring, 48 of whom also had mild sleep apnea. After using the device as instructed for six weeks, time spent snoring at a volume greater than 40dB dropped more than 20% for 87 of the patients. For those with mild OSA, Apnea-Hypopnea Index (a measure of OSA severity) fell 48% in 41 of the patients.
“Obstructive sleep apnea not only impacts sleep quality, but can have other serious health impacts if untreated,” Dr. Malvina Eydelman, director of the Office of Ophthalmic, Anesthesia, Respiratory, ENT and Dental Devices in the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s authorization offers a new option for the thousands of individuals who experience snoring or mild sleep apnea.
Continuous positive airway pressure, or CPAP, is often the go-to treatment for snoring and OSA, but these devices can be cumbersome or outright intolerable for patients who must use them nightly. Stimulation therapy devices have been approved by the FDA for some time, but these products typically require an implant and are intended for severe cases of OSA.
Digital health is no stranger to sleep apnea. The majority of these products have focused on sleep monitoring through wearables, sleep mats and bedside devices, although some others have sought to prevent obstructions by encouraging users to change positions when necessary.




