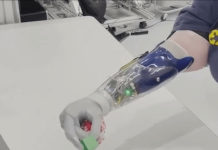
Ekso Bionics (Nasdaq:EKSO) announced that it received FDA 510(k) clearance for its EksoNR robotic exoskeleton.
Richmond, California–based Ekso Bionics designed the EksoNR technology for use with Multiple Sclerosis (MS) patients.
According to a news release, EksoNR is the first exoskeleton device to receive FDA clearance for rehabilitation use in patients with MS, significantly expanding the device’s use to a broader group of patients.
The latest generation of the Ekso Bionics platform, the EksoNR previously received clearance for stroke and spinal cord rehabilitation in 2016, then acquired brain injury (ABI) in 2020. Ekso said it was the first of its kind to receive a stroke indication and stands as the only exoskeleton with indications for ABI and now MS. EksoNR also has CE mark, the company said.
RELATED: Rune Labs scores FDA 510(k) to monitor Parkinson’s symptoms
“As a leader in early-to-market wearable robotic solutions for medical rehabilitation, we are committed to maximizing patient access to our technology,” Ekso Bionics Chair and CEO Steven Sherman said in the release. “With the indications for use now expanded to include MS, the EksoNR has the potential to assist significantly more patients and improve patient mobility. We are excited to see the device benefit MS patients, providing critically needed rehabilitation solutions just as it has for patients suffering from stroke, spinal cord injury and acquired brain injury.”