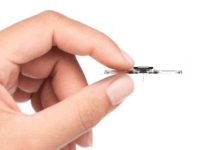
RENOVA iStim™ implantable tibial neuromodulation device is being studied as a minimally invasive, patient-centric solution for the treatment of OAB.
BlueWind Medical, Ltd., developer of the innovative RENOVA iStim implantable tibial neuromodulation device under investigation for the treatment of urgency incontinence alone or in combination with urinary urgency and/or urinary frequency, announced the closing of a $64 million Series B funding round. This round of financing was led by ConvaTec, a global medical products and technologies company focused on therapies for the management of chronic conditions including continence care.
“Proceeds from the financing will be used to support the ramp in our commercial footprint in anticipation of potential FDA marketing clearance for BlueWind Medical’s RENOVA iStim implantable neuromodulation device,” said Dan Lemaitre, Chairman & CEO of BlueWind Medical.
According to the American Urological Association, OAB is a chronic, debilitating condition affecting over 34 million Americans and can lead to urgency urinary incontinence (UUI), the involuntary leakage of urine associated with a sudden compelling desire to void. People diagnosed with UUI have an irreversible condition that substantially impacts their physical and mental health, and is significantly associated with depression, social isolation, obesity, and death.1 2 3
BlueWind Medical completed patient enrollment of the OASIS pivotal clinical study in November 2021 at 23 centers in the United States, United Kingdom, The Netherlands, and Belgium. Interim safety data was recently announced at the SUFU (Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction) Winter Meeting showing no device or procedure related serious adverse events. BlueWind Medical will submit an application for U.S. Food & Drug Administration (FDA) marketing clearance in the United States later in 2022 based on the OASIS pivotal study.
The RENOVA iStim device utilizes neuromodulation to target the nerves that control the bladder. RENOVA iStim is implanted near the ankle in a single short outpatient procedure of approximately 30 minutes utilizing local anesthesia.
“At ConvaTec, we focus on solutions that improve the lives of those enduring chronic conditions like incontinence. We believe BlueWind Medical’s vision for less invasive care with greater customer control will resonate with end-users and providers alike,” said Seth Segel, President & COO, Global Continence Care and Home Services Group of ConvaTec Group Plc.
“The BlueWind team appreciates that ConvaTec and others share our vision in the potential for RENOVA iStim to transform the care of OAB,” concluded Lemaitre.
About BlueWind Medical Ltd.
BlueWind Medical was founded in Israel in 2010 and has an extensive patent portfolio including 15 patent families, 35 filings and 24 issued patents. BlueWind Medical obtained CE Mark for RENOVA iStim in the treatment of overactive bladder in 2016. The OASIS study is being conducted under an Investigational Device Exemption (IDE) from the FDA. RENOVA iStim is not approved for use in the U.S.
For additional information visit BlueWindMedical.com
About ConvaTec Group Plc
ConvaTec is a FTSE 250 global medical products and technologies company focused on solutions for the management of chronic conditions, with leading positions in advanced wound care, ostomy care, continence and critical care, and infusion care. ConvaTec has more than 10,000 colleagues and sell its products and services in over 90 countries. ConvaTec’s vision is Pioneering trusted medical solutions to improve the lives it touches. ConvaTec’s products provide a range of clinical and economic benefits including infection prevention, protection of at-risk skin, improved patient outcomes and reduced total cost of care. Group revenues in 2021 were over $2 billion. To learn more about ConvaTec, please visit http://www.convatecgroup.com