With months still to go, Asensus Surgical has already scored a hat trick this year with a trio of FDA clearances for components of its Senhance robotic surgery system.
The most recent arrived this week, with the agency giving the go-ahead to an upgraded version of Asensus’ artificial intelligence software for the company’s surgical robot. Originally cleared in March 2020, the Intelligent Surgical Unit is designed to give surgeons an enhanced view of the surgical field during laparoscopic procedures.
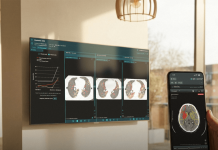
The initial version of the software did so by using machine vision technology to move the surgical robot’s camera in response to a surgeon’s movements and commands and based on the software’s identification of key areas of and objects in the surgical field.
The new upgrade improves on that automated camera control with new zooming and panning abilities and adds 3D measurement and digital tagging features to the system, as well as image enhancements that automatically adjust the brightness and contrast of the camera’s view.
With the 3D measurement feature, surgeons can mark points on the tissue and throughout the abdominal cavity and receive instant readings of the distances between those points, accurate to within a few millimeters. That replaces the traditional reliance on potentially inaccurate estimations or inserted sterile measuring tapes to help surgeons map out staple lines or surgical mesh sizes.
Digital tagging, meanwhile, allows surgeons to virtually flag areas of interest within a patient’s anatomy, making it easier for them to keep track of those areas throughout a procedure. That feature can also be used as a teaching aide, giving surgeons a way to mark up images while guiding trainees through an operation.
“The addition of these pioneering digital capabilities on our existing surgical platform provides real-time intraoperative digital tools to surgeons and underscores our commitment to delivering our vision for performance-guided surgery,” said Anthony Fernando, president and CEO of Asensus Surgical, who suggested that adding the upgraded software to the Senhance system will “enable consistently superior outcomes and a new standard of surgery.”
The ISU software’s clearance comes just about six months after the Senhance platform was given the green light for use in general surgery. Combining highly precise articulating instruments with the automated camera-controlling software and 3D visualization technology, the system can now be used for minimally invasive laparoscopic procedures in and around the abdomen, including reconstructive surgeries to treat reflux, obesity and more.
Between then and now, in late July, Asensus reeled in yet another FDA clearance, this time for its five-millimeter-diameter articulating instruments. When added to the Senhance system, the instruments are able to access typically difficult-to-reach areas of the anatomy, offering up a wider range of motion than the three-millimeter instruments previously used by the system.
The trio of clearances follows a decision at the beginning of the year to rebrand from its original name of TransEnterix. The new Asensus name, Fernando said at the time, reflects the company’s shift from merely a robotics company to a digital surgery company, with a new focus on using AI technologies like machine vision to enhance its robotic surgery systems.
Are you hiring?