Ancora Heart has enrolled the first patient in the CORCINCH-HF pivotal trial. The study is designed to evaluate the safety and efficacy of the AccuCinch Ventricular Restoration System in patients who have symptomatic heart failure with reduced ejection fraction (HFrEF).
The Santa Clara, CA-based company won approval for a pivotal study of the device in June of 2020.
Ancora Heart has enrolled the first patient in the CORCINCH-HF pivotal trial. The study is designed to evaluate the safety and efficacy of the AccuCinch Ventricular Restoration System in patients who have symptomatic heart failure with reduced ejection fraction (HFrEF).
CORCINCH-HF is designed to enroll 400 patients at up to 80 centers worldwide. The study has a design allowing initial analysis of safety and clinical efficacy for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached 12 months of follow-up.
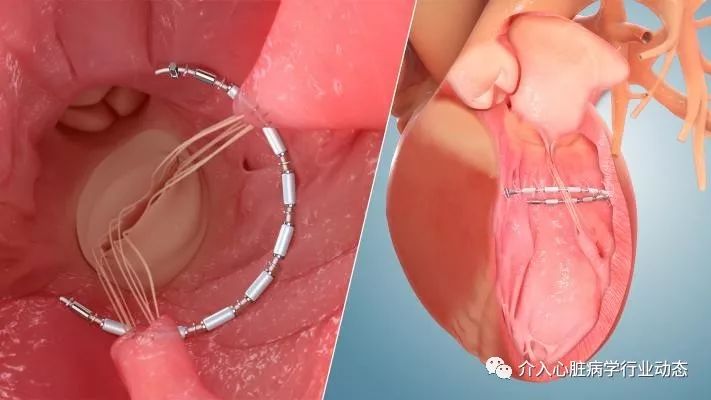
During the AccuCinch procedure, an implant is placed into the mid-left ventricular wall, the implant is then cinched. Once cinched, the implant is intended to reduce the size of the left ventricle, as well as support and strengthen the heart wall to help improve left ventricular function. The AccuCinch System is designed to provide extra stability and support to the left ventricular wall. This may lower stress on the heart and allow improved functional capacity and quality of life for patients.
“Previously collected data on early feasibility implants suggests that the AccuCinch System may afford significant clinical benefits to patients suffering from heart failure with reduced ejection fraction,” Jeff Closs, president and CEO of Ancora Heart, said in a release. “We anticipate that the data from this study will confirm and expand upon these early findings and enable us to ultimately provide an important innovation that may halt or even reverse the progression of heart failure in many patients.”


