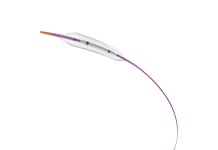
Endogenex has received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to commence a pivotal clinical study of the ReCET System for the treatment of type 2 diabetes (T2D).
The system is aimed at treating adult patients with T2D inadequately controlled by non-insulin glucose-lowering medications. It is designed to target the duodenum’s cellular pathology contributing to T2D progression.
Related: NuvoAir’s Air Next Spirometer is FDA-approved for in-home use
The ReCET Clinical Study is a multicentre, prospective, double-blinded, randomised, sham-controlled trial set to assess the effectiveness and safety of the ReCET System.
Endogenex CEO Stacey Pugh said: “This is a critical next step in advancing the ReCET Procedure as a treatment that addresses the underlying causes of T2D that are not targeted by current diabetes medications.”
For the study, the company plans to enrol up to 350 patients across clinical sites in the US and Australia, with patient enrolment taking place at up to 30 sites in the US and ten in Australia.
The ReCET procedure is an endoscopic, outpatient treatment. It utilises non-thermal pulsed electric fields to initiate the body’s natural regenerative processes.
This aims to restore proper cellular signalling from the duodenum and improve metabolic function, including the regulation of blood glucose levels.
Previous feasibility clinical studies, including REGENT-1 US, REGENT-1 Australia, and EMINENT in the Netherlands, have evaluated the ReCET System.
These studies focused on assessing the safety and efficacy in adults with T2D whose blood glucose levels were inadequately controlled with insulin and non-insulin medications.
Advent Health Diabetes Institute in Orlando, Florida, medical director and ReCET study co-principal investigator Richard Pratley said: “Preliminary results of the ReCET Procedure have been very encouraging, so we look forward to participating in this study and expanding the understanding of how it may fulfil a significant need in the care of this patient population.”