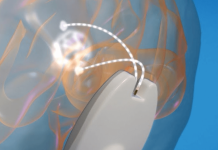
VySpine this week announced it received FDA 510(k) clearance for its VyPlate anterior cervical plate system.
Tallahassee, Florida-based VySpine designed VyPlate to stabilize the anterior cervical spine from C2 to C7 by using unicortical screw fixation at the anterior face the vertebral bodies.
The system has plates and screws that are manufactured from titanium alloy as specified by ASTM F-136. The locking mechanism ensures that the bone screws are fully captured within the system.

RELATED: Visibly receives FDA clearance for online visual acuity test
“The VyPlate ACP System is a unique, elegantly simple system,” CEO Tom McLeer said in a news release. “It offers a great deal of versatility and flexibility while providing ease of use for the surgeon. The VyPlate is intuitive while also being robust and adaptable.”
VyPlate comes in numerous sizes, ranging from a single level to five-level plates. The boy screws can be variable with a high degree of angulation or in a fixed angle to the plate. The system’s bone screws are also available in self-drilling or self-tapping configuration and come in a range of lengths and diameters to provide an optimal fit for unique patient anatomies.
Are you Hiring?