Manus Neurodynamica, which develops and markets products and technologies for neuromotor assessment, has been granted Breakthrough Designation by the U.S. Food and Drug Administration (FDA) for its NeuroMotor Pen (NMP) device.
Breakthrough Designation has been granted for the proposed indication of using the NeuroMotor Pen to support healthcare providers (HCPs) in differentiating between Parkinsonian and non-Parkinsonian tremors in adult patients who are suspected to have Parkinson’s Disease (PD) but have not been diagnosed by other means.
The FDA Breakthrough Device programme is intended to accelerate regulatory approval and help patients receive more timely access to breakthrough technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or debilitating diseases and conditions. This Breakthrough Designation represents a major step towards the technology’s approval in the US market.
Differentiating between a Parkinsonian tremor and other tremor disorders is challenging. Early and correct differential diagnosis is crucial to quickly provide access to best medical treatments and other forms of support. In a community-based study of the accuracy of PD diagnosis, essential tremor (ET), the most common movement disorder, accounted for 48% of the misdiagnoses.
The widespread lack of tremor measurement equipment in the clinic with the ability to detect very subtle tremors is a significant part of the cause for not being able to differentiate between PD and ET.
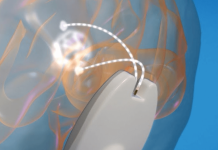
The NeuroMotor Pen is a unique scalable digital solution for the differential diagnosis of ET and PD that combines sensor technologies, built into a digital pen, with software and an analytical engine with Decision Support System. The interface enables users to record movements non-invasively and analyse parameters of minute limb and hand motion, quantifying fine motor skill. These quantified parameters are used as ‘digital biomarkers’ to provide objective information about movement abnormalities, enabling the accurate differentiation between ET, PD and other tremor disorders.
Once registered in the US, the NeuroMotor Pen will be the only device for PD with clinical claims for differential diagnostic decision making. The Company will now agree a study protocol with the FDA in anticipation of a successful US-based clinical trial demonstrating a similarly high specificity and sensitivity in differential diagnostic decision making, as was achieved in European trials.
The prevalence of PD and ET are at least 1.7 and 7 million respectively in the US, and the current annual cost of treatment for PD in the United States is currently estimated at $14.4 billion. As the population ages and life expectancy increases, the number of individuals in the US with PD is projected to double in the coming generation, representing a very substantial existing and growing market for Manus’ NMP technology.
Dr Rutger Zietsma, CEO of Manus, said: “I am delighted that we have been granted Breakthrough Designation for the NeuroMotor Pen to aid in the differential diagnosis of complex forms of Parkinson’s Disease, which is a large and growing cause of long-term illness and disability in the US and the rest of the world.
“Obtaining Breakthrough Designation represents an important milestone in our path to providing a more effective diagnosis for this irreversibly debilitating disease to improve the lives of this underserved patient population. We look forward to building on this achievement by utilising the Breakthrough Devices Programme to facilitate our pursuit of U.S. regulatory clearance for differential diagnosis of Parkinson’s in close collaboration with the FDA.”
Prof Richard Walker, Northumbria NHS Foundation Trust, professor of ageing at Newcastle University, added: “There is great potential for this device in helping us to assess movement and tremor in older people who may have Parkinson’s or other conditions. It can help with making a diagnosis and also with assessing response to treatment.”