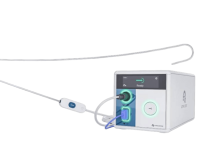
Asahi Kasei’s ZOLL has received approval for its Zenix monitor/defibrillator under the European Union Medical Device Regulation 2017/745 (EU MDR).
The device is designed to increase efficiency and clarity for healthcare professionals across hospital settings and emergency medical services (EMS).
Related: Vesalio earns CE marks for vasospasm, thrombectomy treatments
It features a large, durable touchscreen for rapid access to critical patient information. Its on-the-fly customisation allows real-time adjustments during emergencies, supporting clinical decision-making under pressure.
Zenix incorporates the company’s Real Bag-Valve Mask (BVM) Help and Real Cardiopulmonary Resuscitation (CPR) Help technology.
These features provide real-time clinical feedback to users on ventilation quality and CPR performance.
According to ZOLL, this technology is intended to support both EMS teams and hospital clinicians in making informed decisions and delivering consistent patient care.
ZOLL acute care technology president Elijah White said: “Whether you are a hospital clinician or an EMS professional, the ZOLL Zenix monitor/defibrillator is designed to work the way you work.
“Zenix is our most advanced monitor/defibrillator, and yet it’s incredibly easy to use—virtually all functions of the device are accessible with three screen touches or less. Along with the X Series, R Series and ZOLL M2, Zenix extends ZOLL’s leadership in professional monitors/defibrillators with unique features and intuitive design.”
ZOLL develops medical devices and software solutions in areas such as cardiac monitoring, defibrillation, and CPR feedback.
The company aims to advance patient care in critical cardiopulmonary conditions to help clinicians, EMS professionals, fire professionals, and lay rescuers.
In December 2025, ZOLL introduced its next-generation LifeVest wearable cardioverter defibrillator (WCD) in the US.