Amplifi Vascular announced that it received breakthrough device designation for its Amplifi Vein Dilation System.
The St. Louis-based company also picked up Category B assignment from the Centers for Medicare & Medicaid Services (CMS). It said the two nods mark “a pivotal step” in its mission to address critical healthcare needs.
Related: Imricor wins FDA clearance for MRI-guided EP mapping catheter
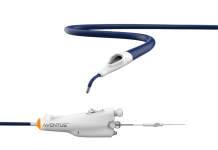
Amplifi Vascular designed its vein dilation system to dramatically enhance vein size and quality prior to arteriovenous fistula (AVF) creation. It aims to deliver more durable, reliable dialysis access and support the shift toward earlier, safer cannulation.
The system is comprised of a wearable, external blood pump with inflow and outflow catheters, plus a controller with a rechargeable battery. It rapidly dilates and preps the vein using well-understood hemodynamics. Removal occurs prior to AVF creation.
According to Amplifi Vascular, data from first-in-human results provided a key factor in securing breakthrough designation. It helps the company streamline the development and review process for its vein dilation system. Concurrent Category B assignment from CMS “further acknowledges” the system’s potential impact, the company said. Amplifi Vascular said it highlights the potential for future coverage and patient access.
The company said its achievements reflect its “dedication to developing impactful medical technologies.” It also demonstrates its potential to improve patient outcomes for those in need of durable, life-sustaining dialysis access.
“This coveted Breakthrough Device Designation reflects the innovative work already completed at Amplifi Vascular, inclusive of a robust first-in-human data from nineteen patients, and also strategically positions us for accelerated development and patient access,” said Sean Morris, Amplifi Vascular CEO. “The ability to engage frequently and efficiently with the FDA, coupled with the Category B assignment from CMS, will significantly streamline our path to market, ultimately benefiting patients in need of life-sustaining dialysis access. With an eye towards market access, we have taken important early steps towards establishing a relationship with CMS and have included important healthcare economics in the context of our clinical study design.”