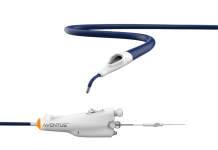
Minneapolis-based Imricor Medical Systems said it won 510(k) clearance from the FDA for its Vision-MR Diagnostic Catheter.
The 9 Fr. catheter is designed for use under real-time magnetic resonance imaging (MRI) guidance for the treatment of heart conditions.
Related: Siemens Healthineers wins FDA nod for helium-free MRI system
The device allows electrophysiologists to visualize cardiac soft tissue with precision and clarity while using what looks, feels, and functions like a traditional ablation catheter, according to Imricor’s website.
It is the company’s first device to receive FDA clearance.
“This is obviously a tremendous milestone for the Imricor team, and I want to acknowledge the outstanding work of the entire team in reaching this achievement,” chair and CEO Steve Wedan said in a news release. “Most of us have worked at companies that have existing medical devices on the U.S. market, and getting a new device on the market is always a big deal. But getting a company’s first device on the U.S. market is an extraordinarily big deal.”
The device was used in the first ischemic ventricular tachycardia ablation ever performed under real-time MRI guidance in an iCMR lab by an Amsterdam University Medical Centre team in the Netherlands in November last year.
“With MRI-guided treatment of heart conditions, we are working towards fewer procedures per patient, hospital admissions, and less medication,” said Dr. Marco Götte, a member of the Amsterdam UMC operating team, on Imricor’s website. “Perhaps MRI-guided treatment of heart disease will become the norm and replace X-ray-driven treatments.”