Atraverse Medical announced that it received FDA clearance for its fully integrated Hotwire transseptal access system.
Hotwire is a novel radiofrequency guidewire left-heart access device. It enables zero exchange left-heart access while acting as a rail for catheter-based therapy systems. Steven Mickelsen, a pioneer in the pulsed field ablation space, invented the Hotwire system with Atraverse co-founder Eric Sauter.
Related: FDA clears Francis Medical’s water vapour ablation treatment for prostate cancer
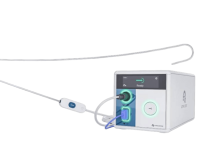
The novel guidewire device initially received the FDA’s green light in May 2024. Now, FDA clearance includes the system’s Hotwire RF (radiofrequency) generator, integrated with the guidewire. The generator mitigates the risk of uncontrolled energy delivery after accessing the left atrium. It can also activate energy within the sterile field to give clinicians more precise control.
Atraverse, which also raised nearly $30 million earlier this year, said it designed the new features to enhance procedural control and workflow efficiency. It offers an end-to-end, zero-exchange, sheath-agnostic solution. The company touts its fully integrated system as the first and only left-heart access system with impedance-guided technology that halts energy delivery upon transseptal crossing. This minimizes unnecessary RF exposure in the left atrium and enables user-controlled energy activation.
The system’s guidewire features a proprietary tip architecture that enhances echocardiographic visualization by 25%. Its reinforced core wire and polymer jacket deliver twice the rail stiffness of leading competitors.
“The FDA clearance of the Hotwire system is a significant milestone for Atraverse — one made possible by the unwavering dedication, ingenuity, and technical excellence of our development team,” said Sauter, co-founder, co-inventor and COO of Atraverse Medical. “After three years of focused innovation, our integrated end-to-end system and intentionally-engineered RF generator now provides a seamless solution to a long-standing clinical challenge — unlocking new opportunities for commercial success and meaningful impact for clinicians and patients.”