AtaCor Medical entered a financing worth up to $75 million to support its U.S. FDA pivotal study.
The study evaluates the company’s parasternal extravascular implantable cardioverter-defibrillator (EV-ICD) system. The EV-ICD aims to treat life-threatening ventricular tachyarrhythmias. Funds add to $28 million brought in last year.
Related: Oath Surgical raises $24 million for surgical AI ecosystem
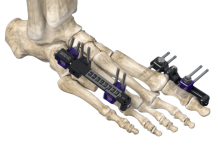
San Clemente, California-based AtaCor designed its EV cardiac pacing and defibrillation lead systems to provide critical cardiac rhythm management (CRM) therapy. They offer benefits without requiring the placement of any hardware inside the patient’s heart. This eliminates some long-term risks associated with intravascular or intracardiac leads.
The platform features the Atala lead and an implantable pulse generator (IPG). Atala, implanted via a small left parasternal incision, goes through the rib space with electrodes placed against the pericardium, outside of the heart and vasculature. The IPG can go in either a lateral or pectoral subcutaneous device pocket, making it a novel option for EV-ICD systems.
AtaCor enrolled the first patient in its ASCEND EV pilot study in March of this year. The company says it completed enrollment and expects to present results in November. Now, it plans to launch the ALARION EV pivotal study in the U.S. and Europe next year. That study would evaluate the EV-ICD and support global regulatory submissions.
“There is a clear and growing need for extravascular ICD systems that combine a straightforward implant procedure with the ability to deliver the full spectrum of tachyarrhythmia therapies using a small pulse generator,” said Rick Sanghera, CEO of AtaCor Medical. “AtaCor is poised to meet that need. We are proud to close this financing round and excited to initiate our pivotal trial next year.”