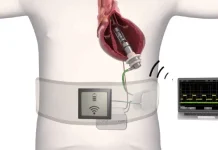
AtaCor Medical launched its clinical study evaluating its extravascular implantable cardioverter defibrillator (EV-ICD) lead system. The San Clemente, California–based company enrolled its first patient in the study following a series of successful initial implantations, which demonstrated the device’s ability to deliver pacing and defibrillation without entering the heart or vasculature.
AtaCor Medical designed the ASCEND EV study to assess the safety and performance of its Atala lead when paired with a variety of commercially available transvenous ICDs. The first cases, conducted at a single site, showed successful sensing and defibrillation of induced ventricular fibrillation using AtaCor’s lead system in connection to a range of commercially available ICDs.
Related: RevBio initiates pivotal Europe trial for its dental implant stabilization product
“Extravascular solutions allow physicians to deliver critical therapy while maintaining the integrity of the heart,” said Dr. Shephal Doshi, ASCEND EV study investigator and executive director of the Heart and Vascular Institute at Providence Saint John’s Health Center & Cardiac Electrophysiology at the Pacific Heart Institute/Cedars Sinai. “AtaCor’s innovative design is an exciting advancement with the potential to elevate the standard for cardiac rhythm management. I am impressed by these first cases, and I look forward to following the clinical progress as more patients are treated.”
The company designed the Atala lead to support anti-tachycardia pacing and defibrillation through a single implant procedure. The system is implanted using a parasternal approach and is compatible with either lateral or left-pectoral pulse generator pocket placement. AtaCor Medical said the implant procedure eliminates some of the complications associated with transvenous leads or the discomfort often linked to other extravascular systems.
“AtaCor is committed to revolutionizing cardiac rhythm management and protecting the integrity of the heart for future cardiac care needs. We are thrilled to have successfully completed this first round of cases, a critical step towards validating AtaCor’s Atala lead,” said AtaCor Medical Chief Medical Officer Martin Burke. “Thank you to the entire AtaCor team for their tireless efforts and our physician partners for their invaluable expertise to achieve this important milestone. We look forward to adding additional clinical sites in the coming months.”
The company plans to expand the study to additional clinical sites in the coming months.
The AtaCor EV-ICD lead system is currently under development for investigational use only and is not yet approved for commercial sale.