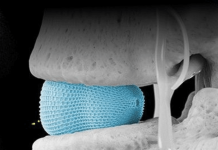
The regulatory study validated Remplir as a safe and effective biological medical device for use in the surgical repair of peripheral nerves and will form a key component of the FDA submission to gain marketing clearance in the US $1.6 billion market.
The findings will also assist the company to further successfully execute its proven strategy to engage high-quality distribution partners.
Related: PixCell Medical gains EU IVDR certification for complete blood count analyser
Orthocell said it remained on schedule to submit its 510(k) application in December CY24, with US FDA clearance expected in Q1 CY25 and commencement of sales soon thereafter.
The study was undertaken with Professor Bill Walsh – the Director of Surgical and Orthopaedic Research Laboratories at the Prince of Wales Hospital in Sydney and University of New South Wales – along with the University of Western Australia’s Winthrop Professor of Orthopaedic Research, Minghao Zheng.
The study was conducted using an established rat sciatic nerve injury model. Repair of surgically transected (severed) nerves was evaluated in 72 rats across three treatment groups.
Orthocell CEO and MD, Paul Anderson, said: “We are thrilled with the results from our U.S. Regulatory Study, validating the superior Remplir clinical outcomes, previously published in a highly regarded, peer reviewed journal. The Study results provide key data for the FDA submission to gain market clearance and start selling Remplir in the U.S. We believe Remplir will redefine the nerve repair market and become an important element in the success of nerve repair surgery.”