InterVene raised $13 million in a Series A financing round to support its Recana catheter system. New investor Treo Ventures and existing investor RiverVest Venture Partners co-led the round. In conjunction with the financing, Brad Vale, Treo Ventures founding general partner, joins InterVene’s board of directors.
Related: SymPhysis Medical secures €2.2m funding for cancer patient device
Redwood City, California–based InterVene plans to use the funds to support the final development of its Recana catheter system. Funds will also support the initiation of clinical cases, regulatory submission and market clearance in the U.S. Additional funds may go toward preparation for initial commercialization and post-market clinical research.
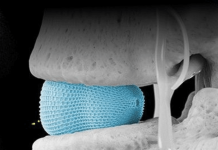
InterVene designed Recana to recanalize and restore patency in chronically obstructed deep veins and venous stents. It particularly targets when recanalization capabilities are limited or when long-term chronic stent maintenance is necessary. Recana utilizes standard fluoroscopy/IVUS-guided interventional techniques.
Vascular specialists already performing deep-vein thrombosis (DVT) treatment and deep venous stenting can easily incorporate Recana, the company says.
“RiverVest and Treo Ventures have strong track records in emerging endovascular fields and InterVene is thrilled to have their support as Recana reaches its final phases of development,” said InterVene CEO Jeff Elkins. “We are excited to work with these seasoned, accomplished teams on such a significant opportunity to help physicians and their patients with venous conditions.”