HEPTA Medical has announced a €5.7m ($6.30m) initial close of its Series A funding round, led by BPI France, to further develop its microwave ablation platform for lung cancer treatment.
The investment will facilitate the US regulatory approval process and the commencement of clinical trials for the platform.
Related: Spectral AI awarded funding to support development of DeepView SnapShot M®
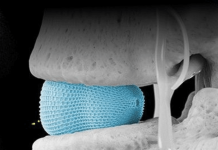
HEPTA’s flexible microwave ablation platform aims to revolutionise early-stage lung cancer management with a non-invasive approach.
The microwave ablation probe is compatible with any lung navigation system and features a unique patented tissue temperature sensor for real-time, in-situ monitoring of ablation zone growth during endobronchial ablation procedures.
HEPTA Medical CEO Dr Thomas Bancel said: “We are grateful for our new investors and excited to bring microwave ablation therapy to new heights. In August, we obtained our first proprietary patent, completing our three patent families under licence.
“We have multiple upcoming milestones, including the release of early preclinical trial results conducted in collaboration with world-renowned centres, which we’ll publish at the CIRSE conference in September.”
The device features AI-powered predictive software and uses procedural CT images and temperature data to provide clinicians with a real-time display of the ablation zone, enhancing the precision and safety of the treatment.
BPI France Life Sciences Investments deputy chief Philippe Boucheron said: “HEPTA’s unique approach to thermal ablation therapy, combined with their dedicated team and cutting-edge technology, positions them at the forefront of transforming lung cancer care.
“We are confident that HEPTA Medical will achieve significant milestones in the near future and are excited to support their continued growth and success.”