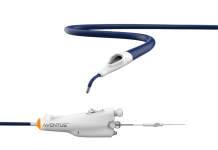
Oasis Medical has announced the launch of its new SOFT PLUG Extended Duration 180-T Tapered Plug, designed to address dry eye syndrome.
The plug features a 0.25mm tapered end, facilitating easy insertion into the punctal opening.
Related: Nexalin secures US patent for deep brain stimulation device
It can be expanded to a 0.6mm diameter at the top end for occluding the canaliculus. The plug is engineered to degrade after approximately 180 days post-insertion.
Crafted from degradable polydioxanone monofilament, the plug is intended for the temporary treatment of dry eye syndrome and associated ocular surface diseases.
It aims to improve the effectiveness of topical medications or ocular lubricants, address contact lens intolerance, and assist in post-surgical dry eye management. The plug also serves as a trial for patients considering permanent occlusion.
Focus Vision & Eye Care optometrist Dr Lacey Thames said: “I have been using the long-duration punctal plugs for over five years. Oasis recently upgraded the long-duration plug to have a tapered edge. The ease of insertion is amazing, and it benefits the patient by allowing a larger diameter plug to be inserted.”
Each sterile SOFT PLUG Extended Duration 180-T Tapered Plug is packaged in pairs, allowing for simultaneous treatment of both canaliculi.
The product is available in boxes containing 40 plugs, or 20 pouches per box, with the reference number 6210.
While the plugs are currently available in the US, Oasis Medical is working towards obtaining the CE mark for broader distribution.
The product was unveiled at the American Society of Cataract and Refractive Surgery (ASCRS) conference in Boston, US.