Biolinq has raised $58m in a financing round to support an ongoing pivotal trial investigating its wearable, non-invasive glucose sensor patch.
Biolinq’s CEO Richard Yang told Medical Device Network: “The pivotal study started in March 2024 and we plan to file for US Food and Drug Administration (FDA) clearance this year.”
Related: Neurava raises $2.3 Million for epilepsy monitoring technology
The funding was led by US investment company Alpha Wave Ventures. The cash adds to a previous $100m raised in a Series B round in 2021.
The glucose-monitoring device sector is growing rapidly as the technology that helps manage the disease becomes more accessible. Continuous glucose monitors (CGMs) are a category of diabetes medical devices that have seen much technological advancement over the past decade. They are wearable devices that use a microneedle inserted into the skin to measure glucose levels in real time. The data is then transmitted to a linked handset or mobile phone.
The global CGM sensor market is expected to grow to more than $5bn in 2033, up from $3.5bn in 2023, according to analysis by GlobalData.
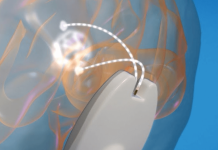
While traditional CGMs are not painful to insert, US-based Biolinq has developed a wearable device that uses an array of electrochemical sensors that the company says are barely perceptible to the wearer. The device, which uses a silicon chip at its core, is up to 20 times shallower than conventional subcutaneous glucose sensors, extending to the dermis only.
The patch is placed on the upper forearm and has a display to alert users when glucose levels are inside or outside of target ranges.
Yang added in a statement: “Our technology approach enables access to a coveted, metabolically active compartment of the skin for biosensing without the use of introducer needles or bleeding.”
GlobalData analysis places Medtronic, Abbott and DexCom as market leaders in the global CGM and insulin pen market. Abbott and Dexcom have their horns locked in an ongoing European patent dispute with infringement claims from both companies relating to CMG patches. In a UK ruling, Dexcom won the case.
Dexcom made history in the space last month when its Stelo system became the first over-the-counter CGM to gain clearance from the US Food and Drug Administration. It is expected to roll out in mid-2024.
Apple meanwhile is rumoured to be developing non-invasive glucose sensor technology for its watches. The tech giant has remained quiet about its diabetes care-related activities.