WhiteSwell announced positive data from 21 patients treated in a trial evaluating its eLym system for fluid removal. The company also announced FDA breakthrough device designation for the system. The agency also accepted it into its Total Product Lifecycle Advisory Program (TAP).
Dr. Jan Biegus presented the data at the Technology and Heart Failure Therapeutics (THT) meeting in Boston. Biegus serves as deputy scientific director at the Institute of Heart Diseases at Wroclaw Medial University in Poland.
Related: Newronika wins FDA IDE clearance for Adaptive DBS System clinical trial
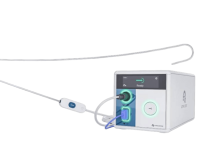
Galway, Ireland-based WhiteSwell designed eLym to treat acute decompensated heart failure (ADHF). The minimally invasive, catheter-based system aims to facilitate the removal of excess interstitial fluid from tissues and organs. It achieves this by supporting the overwhelmed lymphatic system in draining fluid for patients with ADHF.
eLym deploys in the left internal juglar and innominate veins, near where the lymphatic system’s thoracic duct connects. It creates a low-pressure zone, facilitating fluid drainage in conjunction with intravenous diuretics.
“We are encouraged by the clinical results as more patients are treated with the eLym System. WhiteSwell is entering an important phase as we expand our clinical trial sites and look ahead to a randomized controlled trial in the U.S. and other countries,” said Eamon Brady, WhiteSwell CEO. “By supporting the lymphatic system to drain interstitial tissues and organs in conjunction with diuretic therapy, we hope to break the cycle of repeated heart failure hospitalizations for people with ADHF and improve patient outcomes.”