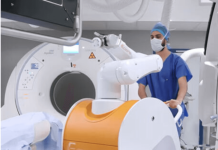
Stereotaxis has received a CE mark in Europe for its minimally invasive endovascular robotic system, GenesisX.
The company has now set its eye on securing marketing clearance in the US and has submitted a 510(k) application with the US Food and Drug Administration (FDA).
Related: Surgical Planning Associates wins FDA nod for mixed-reality hip arthroplasty system
Stereotaxis also says that it plans to gain “regulatory approval for compatible catheters, demonstrate real-world use of the system, enhance compatibility with various X-rays” in the coming months, with a full launch and significant adoption expected by next year.
As per Stereotaxis, GenesisX distinguished itself from other surgical robots with its ease of adoption as the device installation requires minimal structural modifications. The system uses “smaller magnets and incorporates magnetic shielding into its structure in place of the shielding otherwise installed in the walls of the operating room”. Furthermore, the surgical robot does not require floor anchors and specialised power outlets.
The market for surgical robots was worth approximately $7.2bn last year and it is expected to have a strong compound annual growth rate (CAGR) of 15.7%, as per GlobalData analysis. The market growth is propelled by the incorporation of newer technologies such as AI and 5G.
Companies such as EndoQuest Robotics and Omnivision are also working to integrate imaging into surgical robots. In January, the companies partnered to integrate Omnivision’s OCHFA CameraCubeChip image sensor into EndoQuest’s flexible robotic system.
Another device in Stereotaxis is a MAGiC magnetic ablation catheter. In March, the company applied for clearance in both Europe and the US, based on the positive data from a clinical trial. The MAGiC catheter is designed to perform minimally invasive cardiac ablation procedures.