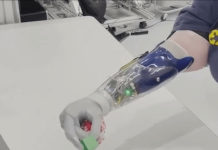
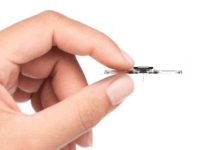
Sensydia has announced the completion of a pivotal study of the Cardiac Performance System (CPS) involving 50 subjects, marking a significant step towards the introduction of a non-invasive device for measuring pulmonary pressure.
The study, conducted at the University of Minnesota, gathered data for the system, which utilises artificial intelligence (AI) and heart sound analysis.
Related: Capitainer secures three US patents
The CPS platform employs ultra-sensitive biosensors to measure critical parameters, such as ejection fraction, cardiac output, and pulmonary pressures, without the need for invasive procedures like echocardiography and right heart catheterisation.
These parameters are said to be critical for assessing heart failure and pulmonary hypertension.
Sensydia president and CEO Anthony Arnold said: “This is Sensydia’s fifth successful study, and we will continue to collect data across leading cardiac care institutions to improve the performance and utility of the artificial intelligence algorithms that power our breakthrough CPS platform.”
Sensydia’s CPS device promises to deliver fast, repeatable, and safe cardiac assessments that can be performed anywhere with minimal training. This contrasts with current methods that are limited to medical facilities and provide only momentary data.
In January 2022, the CPS device received breakthrough device designation from the US Food and Drug Administration (FDA).
Sensydia plans to utilise the data from this latest study to refine the CPS pulmonary pressure algorithms.
UMN Medical School principal investigator and medicine associate professor Tamas Alexy said: “The CPS platform shows promise as a non-invasive alternative to routine echocardiography and right-heart catheterisation. It has the potential to positively impact the way patients with heart failure are monitored and managed, and ultimately to improve clinical outcomes.
“These measurements are essential in the diagnosis and ongoing management of patients with heart failure as well as pulmonary hypertension. We are pleased to help evaluate Sensydia’s platform utilising acoustic sensing technology and advanced AI algorithms, reducing the need for repeat echocardiograms and invasive hemodynamic assessments.”
Last year, Sensydia received a $3m grant to accelerate the development of its CPS.