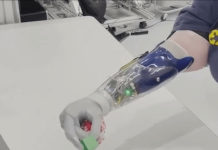
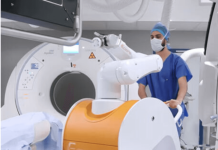
Johnson & Johnson MedTech company Ethicon announced that its Monarch robotic surgery platform received FDA 510(k) clearance.
Ethicon’s Auris Health subsidiary received the FDA clearance for Monarch for endourological procedures. Company officials say it’s now the first and only multispecialty, flexible robotic solution for use in both bronchoscopy and urology.
The company said in a news release that it designed the Monarch robot-assisted surgical platform to enable urologists to reach and visualize areas within the kidney with precision and control. The system offers one platform for supporting both ureteroscopic and percutaneous nephrolithotomy (PCNL) procedures.
“Monarch reduces the complexity of gaining high-quality percutaneous access and aids stone clearance efficiency through simultaneous fragmentation and suctioning of stones with robotic assistance,” University of Southern California professor of clinical urology Dr. Mihir Desai — a paid advisor to Auris — said in the release. “With this platform, many urologists may be willing to expand their practice to include percutaneous access and PCNL procedures, thereby increasing patient access to more effective treatments closer to home.”
The company said several barriers hinder more common use of PCNL, but Monarch offers a way to overcome such barriers with unique, minimally invasive technology. Monarch has been available for robot-assisted bronchoscopy procedures since 2018.
Ethicon said it expects to begin a first-in-human clinical study later this year for the Monarch platform’s endourologic application.
“This latest FDA 510(k) clearance for Monarch delivers on our vision to extend the robotic platform’s capabilities across multiple specialties, enabling hospital systems to target two disease states using one device,” Ethicon Chair Vladimir Makatsaria said. “At Ethicon, we’re committed to driving meaningful innovation that elevates the standard of care for patients and delivers improved solutions that address important clinical challenges for our customers.”