Materna Medical, a company that sells Ob-gyn medical devices, recently raised $22 million in Series B funding. The round was raised by CEO Tracy MacNeal, which stands out because female founders raised just 2% of all venture capital money last year.
Materna Medical recently closed one of the largest funding rounds ever in the women’s Ob-Gyn medical device space. The Mountain View, California-based company, founded in 2010, raised $22 million in Series B funding with lead investment from private equity firm InnovaHealth Partners. The round brings its total funding to date to more than $35 million.
The money will help Materna increase nationwide use of its pelvic health devices, which are both sold directly to women and offered via referrals from providers.
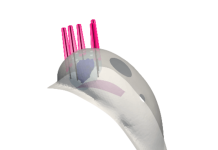
Materna launched its first product, an expanding vaginal dilator called Milli, in 2019. The dilator received clearance from the Food and Drug Administration in December to treat the symptoms of vaginismus and dyspareunia. Vaginismus refers to the involuntarily tensing and contracting of vaginal muscles during wanted penetration, and dyspareunia is the associated pelvic pain that occurs during intercourse and tampon insertion.
Tracy MacNeal, Materna’s CEO, called vaginismus “a poorly understood condition” that is misinterpreted and underdiagnosed. One of the biggest challenges surrounding the condition is that patients and clinicians alike haven’t heard of it.
She said millions of women suffer through pain and anxiety during penetration without knowing they can get treatment. The most common reason patients have vaginismus is that they already have a primary condition that causes pelvic pain that becomes chronic. Common conditions that precipitate secondary vaginismus include menopause, cancer treatment, sexual assault and childbirth injuries.
There is also widespread misunderstanding and misinformation about vaginismus among medical professionals, according to MacNeal.
“Sadly, not much is taught about vaginismus in medical school,” she said. “This lack of information means that patients often suffer with the condition for five years or more and see several clinicians who don’t know how to diagnose or treat it.”
Launched as a consumer wellness product, Milli costs $395. Materna uses healthcare professional referrals in addition to a direct-to-consumer sales approach to sell the device, MacNeal said. More than 130 clinical practices and 3,000 patients have used Milli since its launch.
RELATED: Levita Magnetics Secures $26M to Advance Disruptive Surgical Robotic System
Milli is the only expanding dilator on the market, MacNeal declared.
“The old standard of care for vaginismus was invented in the 1800’s,” she said. “Amielle and Panpac Medical are the only two dilators that have received FDA clearance for the treatment of vaginismus. However, they do not provide the same incremental sizing and ability to expand inside the anatomy at the patient’s discretion.”
Materna’s second device, Materna Prep, is currently being tested in a clinical trial to determine whether it can help first-time mothers achieve less pelvic muscle injuries and a shorter length of delivery during childbirth. The device was developed in collaboration with the Stanford Biodesign program and focuses on the clinical unmet need of pelvic floor lacerations and injury.
“Ninety percent of moms experience perineal lacerations during childbirth — sometimes these are minor injuries, and sometimes they are life-changing, causing permanent disfigurement, sexual dysfunction and long term urinary and fecal incontinence,” MacNeal said. “Additionally, damage to the pelvic floor muscles themselves are common, up to 30% of all childbirth injuries involve muscle damage.”
During the pilot study for Prep, Materna found that the device reduced pelvic floor injury by 60%, according to MacNeal. She said that encouraging finding is why the company is pursuing a larger study involving 15 large health systems, including Michigan Medicine and Texas Children’s Hospital.
Materna will use the money it received in its Series B funding round to help expand the clinical research it is conducting, widen the reach of its devices and prepare for Prep’s market entrance. The funding round, which was raised by MacNeal, stands out as a big score for femtech — female founders raised just 2% of all venture capital money in 2021.