Inspire Medical Systems announced its Inspire therapy has secured its CE mark under the EU’s Medical Device Regulation.
Going into effect in 2021, the tighter regulations in the European Union have been a major challenge for medical device companies. With worries growing that medtech companies would pull products out of the EU because of their inability to comply on time with the MDR, the EU’s government approved extending deadlines to get device designs and quality systems certified to as late as 2028 for some devices.
Related: Restore Medical’s ContraBand device receives FDA’s breakthrough status
“The scale of this transition to a new regulatory framework has proven to be a challenge for medical device manufacturers and the notified bodies who certify them. Given that, Inspire is pleased to reach this critical milestone,” Andreas Henke, Inspire’s EVP and managing director in Europe, said in a news release.
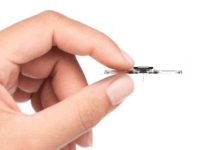
The company’s implantable sleep apnea treatment device has had uninterrupted CE mark approval since 2020. The pacemaker-like neuromodulation device delivers mild electrical stimulation to the hypoglossal nerve, which controls tongue muscles.
“The Inspire team has worked diligently with our notified body in Europe to complete the review process, which included obtaining temporary approval through derogation authorization to continue to deliver Inspire product in several countries,” said Inspire CEO Tim Herbert.
There are two changes to Inspire therapy that are now CE marked under the EU MDR, that were not previously certified under the Active Implantable Medical Device Directive. First, patients in the EU may now undergo full-body MRI scans in the 1.5T MRI environment, provided the scans meet conditions in the company’s MRI guidelines manual. The MDR also provides a CEO mark for the present version of Inspire therapy’s leads with silicone insulation.