IceCure Medical announced the FDA granted clearance for its next-generation single-probe cryoablation system. The XSense cryoablation system with CryoProbes received clearance for all indications for which the company’s ProSense system already holds FDA clearance. That includes minimally invasive cryoablation in general surgery, dermatology and neurology, including cryoanalgesia. It also covers thoracic surgery, ENT, gynecology, oncology, proctology and urology.
Related: CeQur wins FDA nod for mealtime insulin delivery device
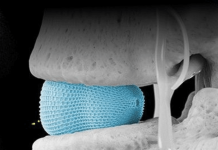
Caesarea, Israel-based IceCure designed the system to destroy tissue by applying extreme cold temperatures. Targets include fibroadenomas, kidney tissue, liver metastases, tumors, skin lesions and warts.
The system utilizes liquid nitrogen, creating large lethal zones for maximum efficacy in tumor destruction. IceCure says the system accelerates recovery while reducing pain, surgical risks and complications. It also has an easy, transportable design for fast, convenient, office-based procedures.
“This latest FDA regulatory clearance further validates the safety and efficacy of our platform cryoablation technology,” said Eyal Shamir, IceCure CEO. “The next-generation XSense system is cleared for the same indications as our flagship ProSense system and we believe it has future potential to address other indications in the U.S. for significant indications with unmet needs.”
IceCure currently seeks clearance for ProSense in the treatment of breast cancer as well. However, the FDA in 2022 denied IceCure’s de novo classification request for ProSense to treat patients with early-stage, low-risk breast cancer. In January of this year, the FDA agreed to reopen the de novo file. An affirmative response from the FDA provided IceCure with a potential pathway to clearance.