Tissium, a medtech company developing biomorphic programmable polymers for tissue reconstruction, has received approval from the U.S. Food and Drug Administration for its Investigational Device Exemption application for its vascular sealant.
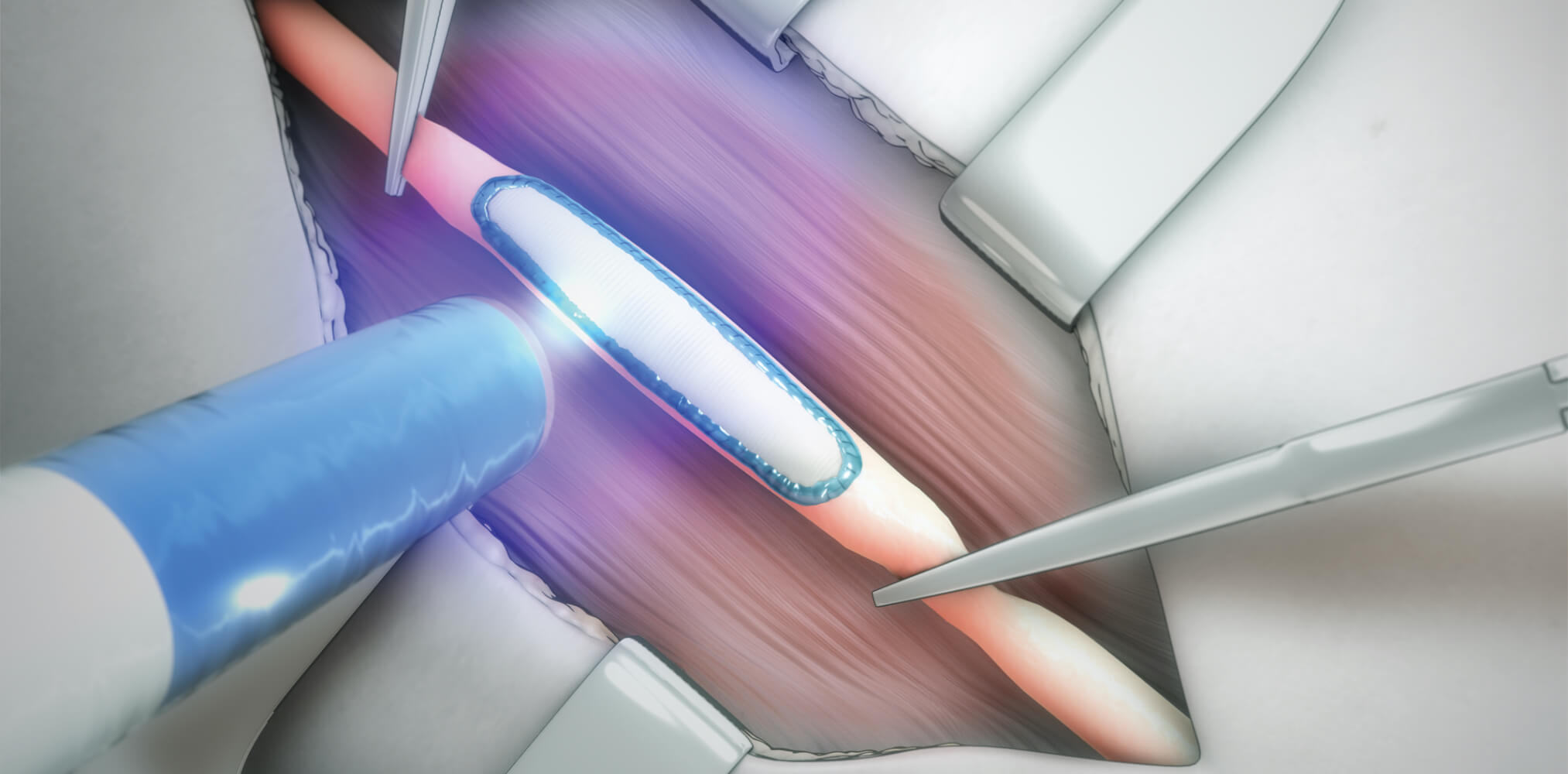
The approval is the most recent validation of Tissium’s efforts to develop a platform of proprietary polymers, activation technologies, and delivery devices, with applications in multiple therapeutic areas.
Tissium’s vascular sealant is designed to address the issue of quickly achieving haemostasis and preventing post-operative bleeding following peripheral vascular surgeries, while also offering biocompatibility and a simple preparation and application process for surgeons. In this application, the configuration of Tissium’s polymer complements sutures as a sealant for a fully effective surgical closure.
Christophe Banel, CEO of Tissium, said: “We are pleased to receive this approval from the FDA as it represents a key milestone that accelerates the development of our vascular indication and triggers the further expansion of our broad platform. We will continue to execute on our strategy to build devices using our core polymer technology and offer applications across multiple therapeutic areas, such as peripheral nerve and hernia repair where we have recently started development.”
The Tissium platform leverages proprietary technology, initially developed at the Massachusetts Institute of Technology (MIT) & Brigham and Women’s Hospital, Harvard Medical School, that serves as the foundation of a family of fully synthetic, biomorphic and programmable polymers. These polymers are designed to be used inside the body as sealants, adhesives, barriers, plugs or as a vehicle for drug delivery, as well as implantable devices created outside of the body using 3D printing technology.
With the approval of the IDE, Tissium will continue expanding the existing platform, leveraging its internal innovation and design team and forging development partnerships to build the stable of devices on its platform.


