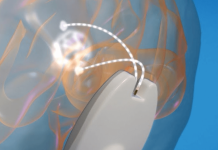
The implant, which stimulates brain circuits via a wireless headset, is designed to have minimal side effects compared to pharmaceutical drugs.
A company developing an implant that stimulates neural circuits in the brain with the aim of treating mental health disorders has successfully conducted the first-in-human test of its device.
Research published on the preprint server medRxiv showed that Motif Neurotech’s prototype microstimulator could safely and effectively stimulate the brain without contact with the organ’s surface.
The miniature pacemaker, approximately the size of a pea, may be able to provide at-home therapy for patients with mental health disorders such as treatment-resistant depression (TRD), a form of major depressive disorder (MDD).
The device is part of a growing wave of implantable brain technology that aims to alter brain circuit activity.
The company says its device is designed to have minimal side effects compared to drugs, which often are used to treat MDD. Some patients do not respond to two or more different antidepressant drugs, resulting in TRD.
RELATED: Meet Tara: the new AI clinical trial matcher for cancer patients
In England and Wales, antidepressant use has been steadily growing, with quarterly antidepressant prescriptions on the NHS hitting 22 million.
Motif says that battery-powered pulse generators and wired leads found in traditional neuromodulation systems lead to several device limitations. Motif’s implant includes features for wireless data and power transmission, designed for use with a wearable headset for therapeutic use at home.
Motif Neurotech co-founder and professor of neurosurgery Dr Sameer Sheth said: “Motif’s minimally invasive neuromodulation device is designed to be implanted in a simple outpatient procedure.”
“This tiny device, which cannot be seen once implanted, provides at-home stimulation that engages brain networks known to treat depression. This is the same brain area activated by transcranial magnetic stimulation (TMS), which is proven to treat TRD but requires frequent clinic visits and usually only provides temporary relief.”
A market model by GlobalData estimates the neuromodulation device market will reach $14.3 bn by 2033.
Questions have been raised about the regulation and trial safety of brain implants. Neuralink, which received approval for human trials of its much-anticipated technology, is under investigation by the US Government for potentially breaching the Animal Welfare Act.