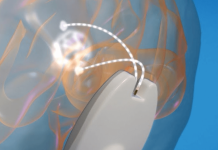
Cardiovascular Systems, Inc. a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that it has received CE Mark for its Diamondback 360® Coronary Orbital Atherectomy System (OAS) and ViperWire Advance® Coronary Guide Wire with Flex Tip (ViperWire Advance with Flex Tip).
“Europe represents a large, underpenetrated market where coronary atherectomy is currently underutilized for the treatment of complex cardiovascular disease. We look forward to collaborating with European physicians to expand the treatment options for patients with severely calcified coronary artery disease.”
The Diamondback 360® Coronary OAS is the market-leading coronary atherectomy device in the United States. Diamondback combines differential sanding and pulsatile forces to treat all calcium modalities, including nodular, eccentric (irregular) and concentric (ring-shaped). This device also features GlideAssist®, which allows for tracking, easier removal and smoother repositioning of the device – particularly in challenging anatomies.

Professor Michael Haude, Director of Medical Clinic, Stadtische Kliniken, Neuss, Germany, EAPCI past-president and former PCR Board member, said, “Interventional cardiologists are increasingly treating older patients with more complex coronary disease. Calcific coronary disease is more prevalent in older patients and those who are smokers, or have diabetes or renal dysfunction. We estimate 12 percent of percutaneous coronary intervention (PCI) patients present with significant calcific coronary disease. For those patients, PCI is associated with a higher risk of procedural complications, as well as poorer long-term results. As clinicians, we are eager to adopt new tools that can improve treatment and outcomes for these patients.”
Professor Nicolas Van Mieghem, Director of Interventional Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, The Netherlands, added, “Calcific coronary artery disease creates a significant challenge to achieving the optimal stent placement needed for best PCI outcomes. By treating all types of calcium, including superficial and deep calcium, the Diamondback 360® Coronary OAS provides one unique solution to optimize stent delivery, expansion and wall apposition to safely support procedural outcomes and ensure long-term clinical results, also in highly complex anatomies. I am excited to add orbital atherectomy to the treatment options for my patients.”
Scott Ward, Chairman, President and Chief Executive Officer of CSI, concluded, “Europe represents a large, underpenetrated market where coronary atherectomy is currently underutilized for the treatment of complex cardiovascular disease. We look forward to collaborating with European physicians to expand the treatment options for patients with severely calcified coronary artery disease.”
About Cardiovascular Systems, Inc.
Cardiovascular Systems, Inc., based in St. Paul, Minn., is a medical device company focused on developing and commercializing innovative solutions for treating vascular and coronary disease. The company’s orbital atherectomy system treats calcified and fibrotic plaque in arterial vessels throughout the leg and heart and addresses many of the limitations associated with existing surgical, catheter and pharmacological treatment alternatives. For additional information, please visit www.csi360.com and connect on Twitter @csi360.
Safe Harbor
Certain statements in this news release are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are provided under the protection of the safe harbor for forward-looking statements provided by that Act. For example, statements in this press release regarding the commercial launch of the Diamondback 360® Coronary OAS and ViperWire Advance with Flex Tip in Europe (including the timing, scope and methods thereof), and the benefits of these products, are forward-looking statements. These statements involve risks and uncertainties that could cause results to differ materially from those projected, including, but not limited to, the reluctance of physicians, hospitals and other organizations to accept new products; the effectiveness of these products; the efforts of our distribution partners in launching these products; the impact of competitive products and pricing; approval of products for reimbursement and the level of reimbursement; general economic conditions; international trade developments; the ongoing COVID-19 pandemic and the impact and scope thereof on CSI, our distribution partners, the supply chain, and physicians and facilities in Europe, including government actions related to the COVID-19 outbreak, material delays and cancellations of procedures, delayed spending by healthcare providers, and distributor and supply chain disruptions; and other factors detailed from time to time in CSI’s SEC reports, including its most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q. CSI encourages you to consider all of these risks, uncertainties and other factors carefully in evaluating the forward-looking statements contained in this release. As a result of these matters, changes in facts, assumptions not being realized or other circumstances, CSI’s actual results may differ materially from the expected results discussed in the forward-looking statements contained in this release. The forward-looking statements made in this release are made only as of the date of this release, and CSI undertakes no obligation to update them to reflect subsequent events or circumstances.
U.S. Diamondback 360® Coronary Orbital Atherectomy System
Indications: The Diamondback 360® Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.
Contraindications: The OAS is contraindicated when the ViperWire® guide wire cannot pass across the coronary lesion or the target lesion is within a bypass graft or stent. The OAS is contraindicated when the patient is not an appropriate candidate for bypass surgery, angioplasty, or atherectomy therapy, or has angiographic evidence of thrombus, or has only one open vessel, or has angiographic evidence of significant dissection at the treatment site and for women who are pregnant or children.
Warnings/Precautions: Performing treatment in excessively tortuous vessels or bifurcations may result in vessel damage; The OAS was only evaluated in severely calcified lesions, A temporary pacing lead may be necessary when treating lesions in the right coronary and circumflex arteries; On-site surgical back-up should be included as a clinical consideration; Use in patients with an ejection fraction (EF) of less than 25% has not been evaluated.
See the instructions for use for detailed information regarding the procedure, indications, contraindications, warnings, precautions, and potential adverse events. For further information call CSI at 1-877-274-0901 and/or consult CSI’s website at www.csi360.com.