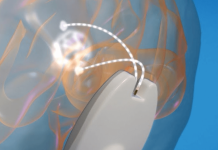
Kardium, a private medical device company that has developed the Globe pulsed field (PF) system for the treatment of atrial fibrillation (AF) using pulsed field ablation (PFA), announced that the first patients have been successfully treated in its PULSAR clinical study.
The international, multicentre study recently received US Food and Drug Administration (FDA) investigational device exemption (IDE) approval in the United States, Health Canada approval in Canada, and SUKL approval in the Czech Republic, to validate the safety and effectiveness of the Globe system.
“We believe the Globe system has the potential to improve outcomes for patients with atrial fibrillation, while also making the procedures easier for physicians.”

The PULSAR study will enrol over 400 patients for treatment at up to 35 sites in the USA, Canada, and Europe. Vivek Reddy (Mount Sinai Hospital, New York, USA) and Atul Verma (McGill University Health Centre, Montreal, USA) are the principal investigators on the study. The first patients were treated this past week at Na Homolce Hospital, Prague, by Reddy, Petr Neužil, and Jan Petru.
The study expands on the successful Globe system first-in-human trial (PULSE-EU) of 69 patients. The initial results of the PULSE-EU study were recently published in JACC: Clinical Electrophysiology. These results demonstrated 100% acute pulmonary vein isolation (PVI) and 100% durable isolation at three-month remapping in patients who received an optimised PFA dose.
“From our experience with the PULSE-EU study, we have found the Globe system can provide a rapid and durable treatment of atrial fibrillation and has so far demonstrated exceptional rates of effectiveness,” said Reddy, “I am excited to begin treating patients in the PULSAR IDE study.”
“The Globe system’s capabilities to deliver rapid PVI and customised PF lesions outside of the pulmonary veins, combined with high-definition mapping and real-time tissue contact maps, allow me to deliver a comprehensive treatment to patients,” Verma stated. “The PULSAR study will expand our knowledge in the exciting and rapidly developing field of PF.”
“The whole team at Kardium is thrilled to be starting the PULSAR study,” said Kardium CEO Kevin Chaplin. “We believe the Globe system has the potential to improve outcomes for patients with atrial fibrillation, while also making the procedures easier for physicians.”
The Globe PF system features the revolutionary Globe catheter with 122 gold electrodes, each of which can map the patient’s cardiac anatomy and electrical activity and deliver PF energy to the heart. The Globe catheter sensors provide proprietary CONTACT maps to identify electrodes in contact with cardiac tissue, helping ensure therapy for atrial fibrillation is effectively delivered.
The PULSAR clinical trial will be used to validate the safety and effectiveness of the Globe system for regulatory approval and commercial sale.