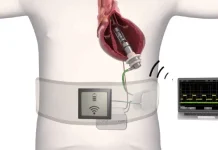
TRIDENT will test the potential survival benefit of initiating Optune concurrent with radiation therapy in patients with newly diagnosed glioblastoma
Preclinical studies demonstrate that Tumor Treating Fields increase sensitivity to radiation therapy and inhibit DNA damage repair
Novocure announced the first patient has been enrolled in its global phase 3 TRIDENT trial, a randomized study in newly diagnosed glioblastoma (GBM) testing the potential survival benefit of initiating Optune concurrent with radiation therapy.
Currently, Optune with maintenance temozolomide is used to treat adults with glioblastoma, following maximal debulking surgery and completion of radiation therapy. However, preclinical studies demonstrate Tumor Treating Fields can be used synergistically with radiation therapy, due to increased tumor sensitivity to radiation therapy, further inhibiting DNA damage repair.
Trident will enroll 950 newly diagnosed GBM patients who, after surgery or biopsy, are candidates for radiation therapy and temozolomide. The experimental group will receive Optune concurrent with radiation therapy and temozolomide for six weeks, followed by Optune and temozolomide. The control group will receive radiation therapy and temozolomide for six weeks, followed by Optune and temozolomide. Patients will continue on Optune for 24 months or until second tumor progression, whichever occurs first.

“We are excited to have begun our TRIDENT trial in newly diagnosed GBM,” said Dr. Ely Benaim, Novocure’s Chief Medical Officer. “The TRIDENT trial represents our commitment to extending survival for GBM patients. We look forward to our partnership with the hundreds of patients who will participate in this study, their families and caregivers, and the nearly 100 leading institutions who have committed to this important research in GBM.”
About Optune
Optune is a noninvasive, antimitotic cancer treatment for GBM. Optune delivers Tumor Treating Fields to the region of the tumor.
Tumor Treating Fields is a cancer therapy that uses electric fields tuned to specific frequencies to disrupt cell division. Tumor Treating Fields does not stimulate or heat tissue and targets dividing cancer cells with specific membrane properties. Tumor Treating Fields causes minimal damage to healthy cells. Mild to moderate skin irritation is the most common side effect reported. Tumor Treating Fields is approved in certain countries for the treatment of adults with GBM and in the U.S. for MPM, two of the most difficult cancer types to treat. The therapy shows promise in multiple solid tumor types – including some of the most aggressive forms of cancer.
Approved Indications
Optune is intended as a treatment for adult patients (22 years of age or older) with histologically-confirmed glioblastoma multiforme (GBM).
Optune with temozolomide is indicated for the treatment of adult patients with newly diagnosed, supratentorial glioblastoma following maximal debulking surgery, and completion of radiation therapy together with concomitant standard of care chemotherapy.
For the treatment of recurrent GBM, Optune is indicated following histologically- or radiologically-confirmed recurrence in the supratentorial region of the brain after receiving chemotherapy. The device is intended to be used as a monotherapy, and is intended as an alternative to standard medical therapy for GBM after surgical and radiation options have been exhausted.
Important Safety Information
Contraindications
Do not use Optune in patients with GBM with an implanted medical device, a skull defect (such as, missing bone with no replacement), or bullet fragments. Use of Optune together with skull defects or bullet fragments has not been tested and may possibly lead to tissue damage or render Optune ineffective.
Use of Optune for GBM together with implanted electronic devices has not been tested and may lead to malfunctioning of the implanted device.
Do not use Optune for GBM in patients known to be sensitive to conductive hydrogels. Skin contact with the gel used with Optune may commonly cause increased redness and itching, and may rarely lead to severe allergic reactions such as shock and respiratory failure.
Warnings and Precautions
Optune can only be prescribed by a healthcare provider that has completed the required certification training provided by Novocure®.
The most common (≥10%) adverse events involving Optune in combination with chemotherapy in patients with GBM were thrombocytopenia, nausea, constipation, vomiting, fatigue, convulsions, and depression.
The most common (≥10%) adverse events related to Optune treatment alone in patients with GBM were medical device site reaction and headache. Other less common adverse reactions were malaise, muscle twitching, and falls related to carrying the device.
If the patient has an underlying serious skin condition on the treated area, evaluate whether this may prevent or temporarily interfere with Optune treatment.
Do not prescribe Optune for patients that are pregnant, you think might be pregnant or are trying to get pregnant, as the safety and effectiveness of Optune in these populations have not been established.
About Novocure
Novocure is a global oncology company working to extend survival in some of the most aggressive forms of cancer through the development and commercialization of its innovative therapy, Tumor Treating Fields. Tumor Treating Fields is a cancer therapy that uses electric fields to disrupt cancer cell division. Novocure’s commercialized products are approved for the treatment of adult patients with glioblastoma and malignant pleural mesothelioma. Novocure has ongoing or completed clinical trials investigating Tumor Treating Fields in brain metastases, non-small cell lung cancer, pancreatic cancer, ovarian cancer and liver cancer.
Headquartered in Jersey, Novocure has U.S. operations in Portsmouth, New Hampshire, Malvern, Pennsylvania and New York City. Additionally, the company has offices in Germany, Switzerland, Japan and Israel. For additional information about the company, please visit www.novocure.com or follow us at www.twitter.com/novocure.
Forward-Looking Statements
In addition to historical facts or statements of current condition, this press release may contain forward-looking statements. Forward-looking statements provide Novocure’s current expectations or forecasts of future events. These may include statements regarding anticipated scientific progress on its research programs, clinical trial progress, development of potential products, interpretation of clinical results, prospects for regulatory approval, manufacturing development and capabilities, market prospects for its products, coverage, collections from third-party payers and other statements regarding matters that are not historical facts. You may identify some of these forward-looking statements by the use of words in the statements such as “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe” or other words and terms of similar meaning. Novocure’s performance and financial results could differ materially from those reflected in these forward-looking statements due to general financial, economic, regulatory and political conditions as well as issues arising from the COVID-19 pandemic and other more specific risks and uncertainties facing Novocure such as those set forth in its Annual Report on Form 10-K filed on February 27, 2020 and its Quarterly Report on Form 10-Q filed on April 30, 2020, as amended to date, with the U.S. Securities and Exchange Commission. Given these risks and uncertainties, any or all of these forward-looking statements may prove to be incorrect. Therefore, you should not rely on any such factors or forward-looking statements. Furthermore, Novocure does not intend to update publicly any forward-looking statement, except as required by law. Any forward-looking statements herein speak only as of the date hereof. The Private Securities Litigation Reform Act of 1995 permits this discussion.