
AliveCor has today announced the receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for healthcare professionals to use the KardiaMobile 6L device to calculate patients’ QTc interval. This FDA action follows the enforcement policy for non-invasive devices issued by the agency in March 2020 that allowed for manual QT measurement by healthcare professionals during the COVID-19 pandemic.
With this new FDA-clearance healthcare professionals can use the KardiaMobile 6L device to obtain an electrocardiogram (ECG) which they can use to manually measure their patients’ QT interval. Obtaining an ECG through this device is fast, easy, and convenient as it can be done by the healthcare professional in the office or anywhere remotely by the patient. KardiaMobile 6L is the first and only hand-held ECG device that is FDA cleared for measurement of QTc.
“Patient safety is paramount, and this is why we are proud to offer physicians the ability to monitor QTc through the convenience and quality of our device,” said Priya Abani, CEO, AliveCor. “It is our hope that this important FDA clearance will help healthcare professionals identify and save patients from this potentially life-threatening condition.”
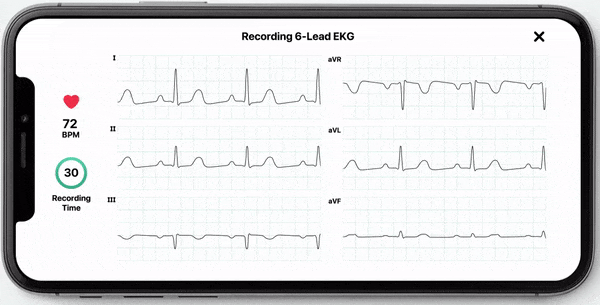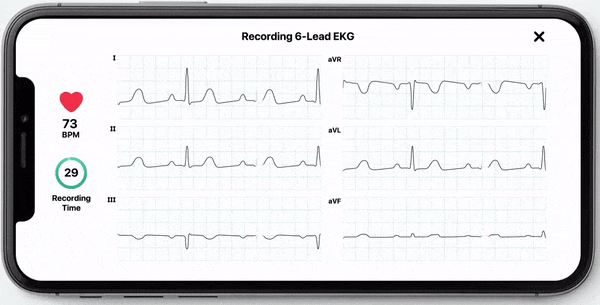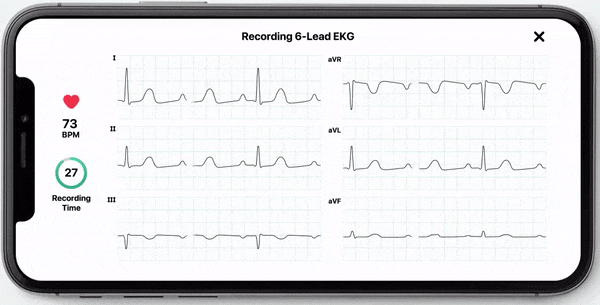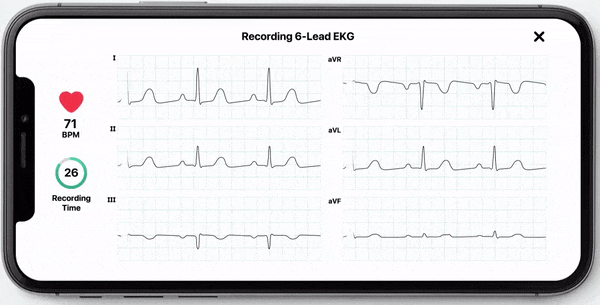
In addition to allowing medical professionals to calculate QTc, AliveCor now offers InstantQT, a service that measures QT intervals quickly and accurately through FDA-cleared, ECG processing software. The InstantQT service can assist healthcare professionals in detecting potentially dangerous QT prolongations in patients.
Are you hiring?




