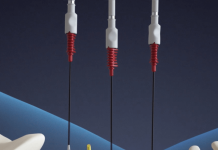
Leadoptik announced the successful first-in-human clinical use of its FDA-cleared Last Inch Assessment (LIA) system, the company tells MassDevice. The first-in-human use took place at the University of Chicago Medical Center. It comes on the heels of FDA 510(k) clearance for the system designed to improve diagnostic accuracy during lung biopsy procedures.
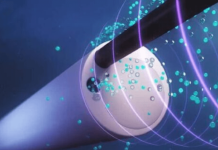
LIA combines imaging and biopsy into a single, integrated device. Compatible with all conventional bronchoscopes, the system offers real-time, high-resolution imaging to visualize deeper into tissues.
Related: Spineology launches innovative patient-specific spinal implant
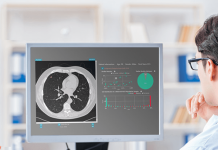
Leadoptik said the first-in-human case utilized LIA’s integrated optical imaging. This feature provides real-time, depth-resolved insights at the point of tissue sampling. The company designed the technology to help physicians confirm that biopsy tissue is collected from the intended target. Leadoptik says this addresses a longstanding challenge in lung cancer diagnosis.
Dr. D. Kyle Hogarth, professor of medicine and director of interventional pulmonary and advanced bronchoscopy at the University of Chicago, said LIA integrated seamlessly into the clinical workflow and provided real-time, intra-tool lesion confirmation during tissue sampling. Hogarth called the initial accuracy “highly encouraging” as it reinforced confidence at the point of biopsy.
“Treating our first patients marks a defining moment for Leadoptik,” said Reza Khorasaninejad, CEO and co-founder of Leadoptik. “After years of development and encouraging preclinical data, we are now translating this technology into early clinical experience. Our goal is to help clinicians make more confident decisions and help patients get answers sooner. In the first six patients, LIA was able to differentiate nodules from healthy tissue in real time, something that has not been possible before.”