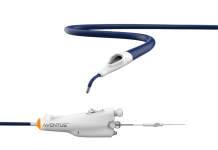
Smith+Nephew has agreed to acquire Integrity Orthopaedics, a US-based developer of the Tendon Seam rotator cuff repair (RCR) system, for up to $450m.
Under the agreement, Smith+Nephew will purchase Integrity Orthopaedics for an upfront cash payment of $225m, with further performance-linked payments of up to $225m payable over the next five years.
Related: Boston Scientific to acquire urinary incontinence developer Valencia Technologies
The acquisition is expected to strengthen Smith+Nephew’s shoulder repair portfolio and is part of the company’s plan to grow its presence in sports medicine, with a focus on RCR systems. It is anticipated to close before the end of January 2026.
RCR is a significant and expanding field, with 500,000 procedures conducted every year in the US. The estimated market value currently stands at $875m. Traditional repair techniques have shown structural failure rates between 20% and 40%, highlighting the ongoing need for improved clinical solutions.
Tendon Seam is designed to address these unmet needs. The system uses individually locked stitches, continuous suture, patented micro anchors, and an integrated instrument to provide robust repairs and faster patient recovery with lower re-tear rates.
Initial clinical experience suggests that Tendon Seam may lead to shorter sling use and procedure times when compared to conventional techniques.
The system obtained 510(k) clearance in 2023 and is approved for reattaching soft tissue to bone, including ligaments, joint capsules, and tendons.
Integrity Orthopaedics was established in 2020 in Maple Plain, Minnesota, US. The company’s founders include Tom Westling, also a co-founder of Rotation Medical, which developed the REGENETEN Bioinductive Implant acquired by Smith+Nephew in 2017.
Smith+Nephew sports medicine president Scott Schaffner said: “Smith+Nephew now has an unrivalled portfolio for shoulder, including a powerful next-generation RCR platform to complement market-leading biological augmentation, the newest shoulder arthroplasty system, and proven solutions across shoulder instability.
“We welcome the Integrity team, including those involved with our prior successful acquisition of Rotation Medical, and look forward to working together again to offer customers and their patients this exciting technology.”
Smith+Nephew’s shoulder solutions portfolio includes technologies that offer a comprehensive suite for both mechanical repair and replacement of the shoulder.
In July 2024, Smith+Nephew received 510(k) clearance from the US Food and Drug Administration (FDA) for its CATALYSTEM Primary Hip System.