Endologix LLC today announced the first implant of its ALTO® Abdominal Stent Graft in Canada following recent approval from Health Canada. ALTO was also recently approved for commercial sale in Argentina.

“We are excited to extend the global reach of ALTO, which is now available in Europe, New Zealand, Canada and Argentina, as well as the U.S.,” said Prof. Matt Thompson, chief medical officer of Endologix. “ALTO fulfills an unmet clinical need, offering a highly differentiated endovascular treatment option for patients with AAA and offers the ability to treat the largest proportion of patients within the approved indications for use.”
The first case in Canada was performed on May 4 by Dr. Ghislain Nourissat at Hôpital Saint-François-d’Assise in Quebec. ALTO was used for this patient within the approved indications for use despite the presence of hostile neck anatomy and narrow access vessels.
“As an early adopter of polymer technology, I have seen how ALTO’s custom sealing technology provides a precise patient-specific seal for a diverse range of anatomies,” Dr. Nourissat said. “With this technology, as well as its low profile and short neck indication, I believe ALTO has the potential to become the standard in endovascular aneurysm repair.”
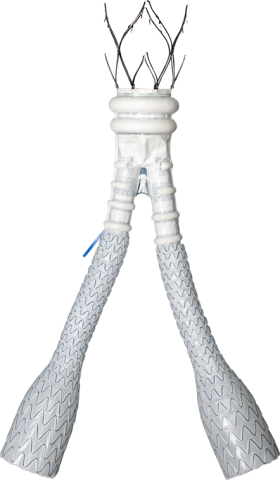
About Abdominal Aortic Aneurysm (AAA)
AAA occurs when a portion of the abdominal aorta bulges into an aneurysm because of a weakening of the vessel wall, which may result in life threatening internal bleeding upon rupture. Patients diagnosed with a AAA can be treated with open surgical repair or via less invasive Endovascular Aneurysm Repair (EVAR).
About the ALTO Abdominal Stent Graft System
Featuring a unique, patented, sealing technology, the ALTO Abdominal Stent Graft System is the latest generation in polymer-based therapies for AAA patients. ALTO utilizes a low-profile delivery system and, unlike standard EVAR devices, features an exclusive conformable sealing ring that molds in-situ to the patient’s specific aortic neck anatomy.
About Endologix’s Product Portfolio
Endologix has a therapeutic portfolio designed to treat diseases that have clinically relevant unmet needs. The company’s products are designed to treat a wide spectrum of vascular disease through abdominal aortic aneurysms to lower limb peripheral vascular disease. Excellent clinical outcomes are achieved through meticulous attention to product design, manufacturing, and training, all backed by industry-leading clinical evidence. Endologix’s AAA products are built on one of two platforms: traditional minimally invasive EVAR and endovascular aneurysm sealing (EVAS), which is an investigational product. The company’s current commercial EVAR products include the AFX®2 device and the ALTO® Abdominal Stent Graft System. Through the recent acquisition of PQ Bypass, Endologix is developing a new approach to treat percutaneous femoral-popliteal bypass. The Detour platform, which is an investigational product that has been designated by the U.S. Food & Drug Administration (FDA) as a Breakthrough Device, consists of the TORUS Stent Graft and the PQ Crossing Device.
About Endologix
Endologix LLC is a California-based, global medical device company dedicated to improving patients’ lives by providing innovative therapies for the interventional treatment of vascular disease. Endologix became a private company wholly owned by Deerfield Management on October 1, 2020. Prior to that, Endologix had been a publicly traded company. The company has offices and manufacturing sites in Irvine and Santa Rosa. To learn more about Endologix, please visit http://www.endologix.com/.




