Phantom Neuro today announced it received FDA breakthrough device designation and Targeted Acceleration Pathway (TAP) designation for its minimally invasive neural interface Phantom X.
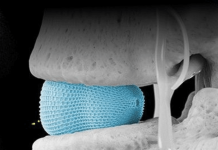
The Austin, Texas-based neurotech company designed Phantom X as a minimally invasive neural interface that provides intuitive control of prosthetics and robotic devices. The FDA breakthrough designation and TAP designation allow the company to facilitate early and strategic communication with the FDA to help speed up the pathway to commercialisation.
Related: Vitestro launches Aletta robotic phlebotomy device for blood draws
Phantom X is implanted just beneath the skin in an outpatient procedure that is accessible by over 70,000 qualified surgeons, according to Phantom Neuro. The platform uses algorithms to decode natural human movement and provide a near-human experience when controlling prosthetic limbs and assistive technologies.
In a recent study, the company said Phantom X achieved 94% accuracy in decoding real-time gestures across 11 essential hand and wrist movements. Phantom Neuro anticipates additional trials in 2025.
“Receiving both of these certified designations from the FDA is a tremendous validation of our work,” said Connor Glass, founder and CEO of Phantom Neuro. “These recognitions validate our technology and reflect our commitment to creating scalable, real-world solutions that restore functionality and independence to amputees and those with functional disabilities. Our goal is to bring Phantom X to patients faster, and achievements like this accelerate our clinical and regulatory processes.”