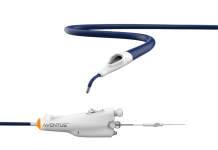
US-based Protaryx Medical has completed a first-in-human trial of its transseptal puncture (TSP) device.
The company’s trial enrolled five patients, with the TSP procedure carried out using its device at the Sanatorio Italiano hospital in Paraguay under approval from the Research Ethics Committee of the Paraguayan Institute of Social Studies.
The company said the trial met all procedural success endpoints, with targeted TSP achieved with minimal fluoroscopic exposure and no adverse events. All patients in the trial were discharged within 24 hours of their procedures being completed, with no complications reported.
Related: FemPulse wins FDA IDE for for overactive bladder neuromod
A process used in structural heart interventions that includes mitral valvuloplasty and radiofrequency ablation, TSP allows physicians to access the left atrium and ventricle of the heart through the interatrial septum.
According to Protaryx, the device’s features include radiofrequency guidewire technology compatible with standard electrosurgical generators and an extendable, echogenic, atraumatic probe. These features make the technology more intuitive and accessible for operators of all experience levels.
Dr Gagan Singh, an interventional cardiologist at UC Davis Health who supervised the TSP procedures undertaken in the trial, commented: “The device substantially lowers the technical learning curve for targeted TSP due to its simplistic design, rapid echo visualisation of septal contact, and ease of negotiating the septum until the desired target is acquired.”
Protaryx Medical CEO David Mester said: “This achievement marks a pivotal step forward for Protaryx.
“We are excited to advance toward 510(k) submission and bring our innovative device to market, where it has the potential to redefine transseptal procedures.”
In 2020, Protaryx raised $8.3m from non-dilutive grants and a Series A funding round led by Ajax Health.
According to GlobalData analysis, the global interventional cardiology market is forecast to reach a valuation of around $33.47bn by 2033.
In other structural heart news, Abbott commenced the first subject procedures last month with its transcatheter aortic valve implantation (TAVI) system to treat symptomatic severe aortic stenosis.
During the same month, Medtronic reported new clinical data from the study of its Evolut transcatheter aortic valve replacement (TAVR) system, with the Optimize PRO clinical study demonstrating positive procedural and clinical outcomes when using the cusp overlap technique (COT) with the Evolut PRO and PRO+ devices.