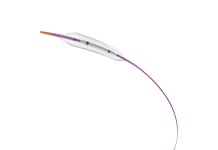
Si-Bone announced the first patient procedures with its FDA breakthrough device, the iFuse Torq TNT implant system. Santa Clara, California-based Si-Bone designed TNT to address the anatomic and biomechanical challenges of pelvic fragility fractures. It particularly helps in patients with poor bone quality, offering a significant advancement over traditional cannulated screws.
Related: Ocutech launches device to aid with vision loss after stroke and brain injury
TNT received FDA 510(k) clearance and FDA breakthrough device designation in August. Si-Bone said it’s the first 3D-printed transiliac-transsacral screw cleared for market use in the U.S. It features a pelvis-specific design to improve initial fixation and reduce the risk of screw backout.
Dr. Edward Westrick (Allegheny General Hospital, Pittsburgh), Dr. Reza Firoozabadi (Harborview Medical Center, Seattle), Dr. J.D. Black (Kadlec Regional Medical Center, Richland, Washington) and Dr. Brian Cunningham (Methodist Hospital — HealthPartners, St. Louis Park, Minnesota) were among the first to perform procedures with TNT.
Black said its streamlined instrumentation offered “excellent fixation” and quick, precise implantation. Cunningham touted its 3D-printed porous surface facilitating osseointegration. He said that leads to better long-term outcomes for older, osteoporotic patients.
“We are thrilled with the successful completion of these initial procedures using our iFuse Torq TNT system,” said Laura Francis, Si-Bone CEO. “This breakthrough technology marks a significant step forward in addressing the unmet clinical needs of complex pelvic fragility fractures. By providing a solution that improves both surgical efficiency and patient recovery, we are further expanding our leadership in the sacropelvic space.”