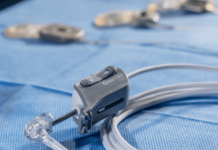
Signum Surgical has secured De Novo clearance from the US Food and Drug Administration (FDA) for its BioHealx technology for anal fistula treatment.
BioHealx is a single-use, bioabsorbable implant. It is designed for a minimally invasive procedure to treat anal fistula, a significant development in colorectal disease management.
Related: FDA issues 510(k) approval for Epica’s See Factor CT3 system
The device has been developed in partnership with colorectal surgeons and aims to close the internal opening of the fistula tract, prevent recurrence, promote healing, and protect patient continence.
The FDA’s De Novo classification and clearance follow a comprehensive clinical trial completed last year.
Conducted across multiple centres in Hungary, the trial involved 32 adult patients and aimed to assess the safety and efficacy of BioHealx in a real-world setting, providing a new solution for patients who have suffered from recurrent anal fistula.
Patients involved in the trial had previously experienced at least one failed treatment for recurrent anal fistula. The final follow-up assessments for the patients ranged from 13 to 40 months.
Signum Surgical co-founder and CEO Moshe Zilversmitsaid: “We are proud to develop BioHealx, now an FDA De Novo-cleared medical device for the treatment of anal fistula. This is a significant milestone for our business and an important step to bring this novel treatment to market for the benefit of patients, surgeons, and the healthcare system.
“We are currently in discussions with potential strategic commercialisation partners to explore how to rapidly and efficiently make the BioHealx solution available to patients.
“FDA clearance for BioHealx, which is protected by our growing intellectual property portfolio, continues our strong momentum in addressing the unmet clinical need for the treatment of anal fistula.”